Scheme 3.
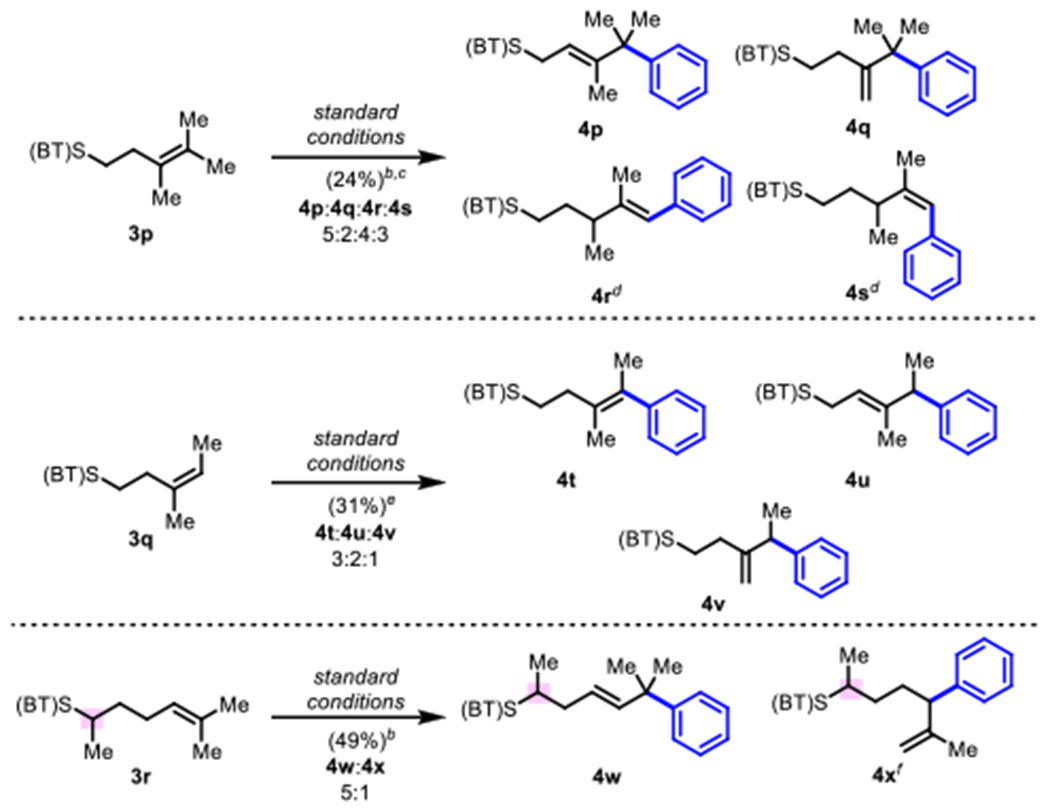
Results with Tri- and Tetra-substituted Olefinsa
aReaction conditions: alkene (0.1 mmol), phenylboronic acid (0.14 mmol), BQ(0.15 mmol), Pd(OAc)2 (5 mol%), DMSO (1 mL), 45 °C, 3 h, unless otherwise noted; isolated yields. bRun at 65 °C and in DMSO (2 mL). cIsolated as a 5:2:4:3 mixture of isomers; 50 mM at 65 °C. dIsomers separated by HPLC. eIsolated as a 3:2:1 mixture of isomers; isomers separated by HPLC. fObserved by NMR.
