Scheme 8.
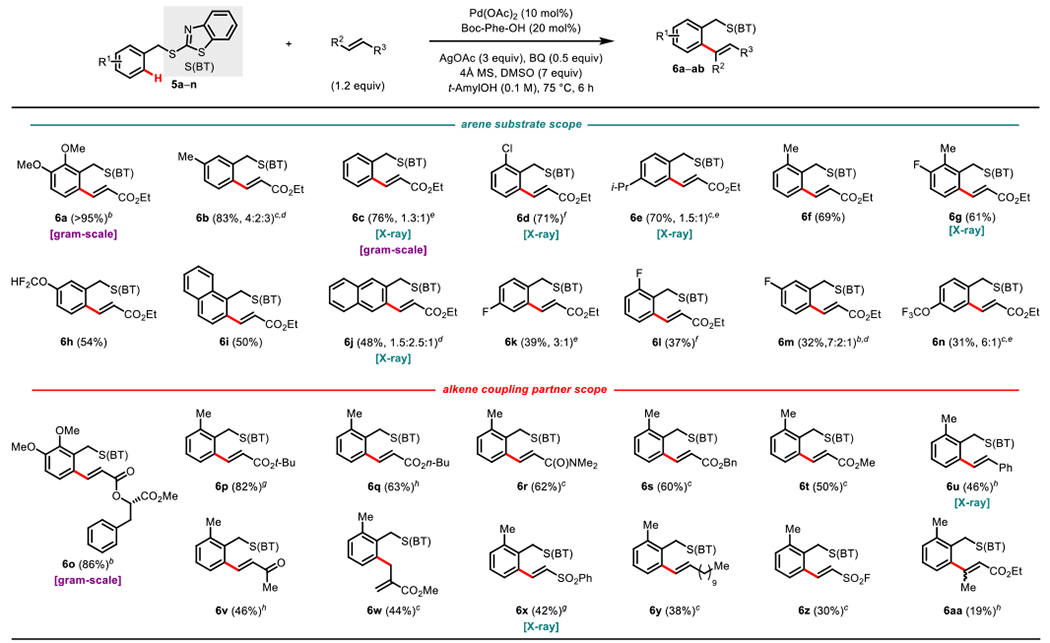
Scope for (BT)S-directed C-H Functionalizationa
aReaction conditions: 5a–aa (0.10 mmol), Alkene (1.2 equiv), AgOAc (3 equiv) Benzoquinone (0.5 equiv), Pd(OAc)2 (10 mol%), tAmylOH (1 mL), Boc-L-phenylalanine (0.2 equiv), DMSO (7 equiv), 4Å molecular sieves, 75 °C, air, 6 h. Percentages refer to isolated yields. b110 °C, nBu4NPF6 (2 equiv), 2h. cAlkene (2.2 equiv). dRatio of mono-, mono’-, and bis-olefinated products, respectively, with the major product depicted. Mono- and mono’-olefinated products isolated as a mixture. eRatio of mono- and bis-olefinated product, respectively, with the major product depicted. fAlkene (2.2 equiv), g24h. hAtkene (2.2 equiv), 24 h.
