Abstract
Background:
Based on the role of bone marrow (BM) stem cells in regeneration of endometrium, refractory cases of Asherman's syndrome (AS) and endometrial atrophy (EA) may benefit with BM-derived intrauterine stem cell instillation. Aims and Objectives: To evaluate the role of BM-derived autologous stem cell therapy in endometrial regeneration and restoration of menstruation and fertility in refractory cases of AS and EA.
Setting:
This study was conducted at a tertiary care center.
Design:
This was a prospective, single-arm longitudinal study.
Materials and Methods:
Twenty-five cases with refractory AS or EA were included. BM-derived mononuclear stem cells were instilled into the subendometrial zone followed by oral estrogen therapy for 3 months. Menstrual flow and endometrial thickness (ET) were assessed at 3, 6, and 9 months and 5 years.
Results:
Statistical analysis was carried out using statistical software STATA version 12.0. Mean prestem cell transfer ET (mm) was 3.3 ± 1.0. At the end of 3 months, there was a significant increase in ET (mm) to 5.1 ± 1.9 (P = 0.001), but there was no significant change at 6 months (5.6 ± 1.5; P = 0.164), at 9 months (6.1 ± 1.7; P = 0.135), or at the end of 5 years. Six of the seven amenorrheic patients resumed menses. Three patients had a successful pregnancy outcome.
Conclusion:
Intrauterine stem cell treatment is a promising novel approach for refractory cases of AS and EA.
KEYWORDS: Asherman's syndrome, bone marrow, endometrial atrophy, stem cells
INTRODUCTION
Global burden of infertility has steadily increased over years, with an estimated 48.5 million couples affected worldwide.[1] Among the various causes of female infertility, Asherman's syndrome (AS) and endometrial atrophy (EA) contribute to a significant number of cases.
AS is the partial or complete obliteration of the uterine cavity with adhesions, resulting in scanty menses, amenorrhea, infertility, or recurrent pregnancy loss. Incidence varies between 2% and 22% in infertile women.[2,3] Risk factors include endometrial curettage, myomectomy, intrauterine contraceptive device (IUCD) insertion, and infections particularly genital tuberculosis (TB).[2] The treatment of choice of AS is hysteroscopic adhesiolysis. Following adhesiolysis, various preventive measures such as IUCD insertion, intrauterine balloon stent, Foley's balloon catheter insertion, and anti-adhesion barriers such as hyaluronic acid or medical treatment such as estrogen or sildenafil are used to prevent recurrence.[4] In spite of all these measures to prevent adhesions, high recurrence rates are noted particularly in moderate and severe AS (21% and 50%, respectively).[5]
Adequate endometrial thickness (ET) plays a key role in embryo implantation. ET <5 mm is associated with lower chances of pregnancy.[6] Reason for atrophic endometrium could be idiopathic or uterine surgeries, endometrial curettage, ovarian stimulation with clomiphene citrate, prolonged use of progesterone, or combined oral contraceptive pills.[7] This condition is prevalent in 0.5% of infertile women undergoing assisted reproductive treatment.[6] For treatment of atrophic endometrium, various treatment options are available such as prolonged estrogen administration, vaginal sildenafil, Vitamin E, pentoxifylline, and luteal phase gonadotropin-releasing hormone-agonist supplementation, but none have proved very effective.[6]
Adult stem cells are rare undifferentiated cells with functional properties present in most adult tissues and organs in the body. Their role is to replenish cells lost through cellular turnover and tissue damage. Basal layer of the endometrium also harbors these stem cells which regenerate the functional layer of endometrium. The role of stem cells in endometrial regeneration is not limited to local endometrial progenitor cells; hematopoietic and nonhematopoietic bone marrow (BM)-derived stem cells are also recruited to the endometrium in response to injury.[8] Based on this role of BM stem cells in regeneration of endometrium, refractory cases of AS and EA can potentially benefit with intrauterine stem cell instillation.[9]
In view of high recurrence rate of adhesion formation posthysteroscopic adhesiolysis, despite preventive measures and lack of effective evidence-based therapy for EA, the role of stem cells in endometrial regeneration needs to be further explored in these cases. Stem cell therapy may provide us with a solution to this problem where currently the only option left is surrogacy or adoption. Existing literature has very few human studies on the role of stem cell therapy in endometrial regeneration, and all these studies were performed on a very small group of patients.[8,10,11] This is the reason why this study was conducted.
The objective of this study was to evaluate the role of intrauterine autologous stem cell implantation in regeneration of the endometrium and restoration of menstruation and fertility in refractory cases of AS and atrophic endometrium.
MATERIALS AND METHODS
This was a prospective, single-arm longitudinal study, approved by the Institutional Committee for Stem Cell Research and Therapy and Human Ethics Committee of our institute and funded by our institute research grant. The trial was registered with CTRI – CTRI/2013/08/003896.
The study was conducted on infertile women (primary or secondary infertility) of reproductive age group (aged 24–38 years) with AS (Grade I–IV) or EA (ET <5 mm) not responding to standard treatment options (hysteroscopic adhesiolysis followed by estrogen treatment in patients with AS and estrogen treatment in patients with EA). Women with active genital TB, those with chronic debilitating or hematopoietic diseases affecting the BM, and those not willing to participate were excluded from the study.
Twenty-five patients were enrolled (12 with AS and 13 with EA). Four patients with AS (Grade 1) and all 13 patients with EA were seeking in vitro fertilization (IVF) treatment for tubal (16 patients) or male factor (two patients). In these 17 patients, AS or EA was diagnosed on pre-IVF evaluation. Informed written consent was obtained from each participant before the procedure. Detailed history was taken and examination was performed. Hormone profile (follicle-stimulating hormone and luteinizing hormone) was done. Diagnostic hysteroscopy was performed on all 25 patients in proliferative phase of menstrual cycle in operation theater under sedation. In patients with AS, hysteroscopic grading of adhesions was assessed using the European Society of Hysteroscopy Classification of intrauterine adhesions.[12]
Following this, BM aspiration was performed under local anesthesia in hematology daycare center of our institute. Under strict aseptic precaution, BM (30 ml) was aspirated from iliac crest using a disposable BM aspiration needle (Jamshidi, 11 G) and collected in heparinized syringes. Samples were immediately transported to our stem cell facility in sterile plastic tubes in a transport incubator for adult stem cell harvesting.
Preparation of hematopoietic stem cell
The isolation of mononuclear cells (MNCs) was done by Ficoll density separation method. BM was diluted in 1:3 ratio with phosphate-buffered saline (PBS) and layered over lymphocyte separation medium and centrifuged at a speed of 800 g for 25 min.[13] MNCs (buffy coat) were aspirated with a 10 ml disposable pipette and washed thrice in heparinized normal saline (NS)/PBS.[14] Finally, MNCs were suspended in 3 ml heparinized NS.
The MNCs obtained were evaluated for:
Total cell count: Cell numbers were assessed by counting the cell in the Neubauer chamber under microscope
Cell morphology: MNC were stained with Giemsa stain and observed under microscope
Viability: Trypan blue dye exclusion test was done to know the percentage of live cells.
All these procedures were performed in the stem cell laboratory at our center. The same day, the patient was taken up for stem cell implantation. Preprocedure diagnostic hysteroscopy and stem cell implantation were performed by the same surgeon in all the cases.
Stem cell implantation
All 25 patients underwent stem cell implantation between January 2013 and January 2014. The procedure was conducted under intravenous sedation with antibiotic cover (single dose of 1 g cefazolin IV). The patient was laid in lithotomy position. A transvaginal ultrasound (TVS) probe with a sterile disposable probe cover, and a guide attached to it was used to locate the subendometrial zone. After locating the subendometrial zone on ultrasound (Wipro GE Voluson), an ovum pick-up needle (Cook No. 17) was introduced vaginally via the lateral fornix and stem cells were implanted in the subendometrial zone transmyometrially. A volume of 3 ml of MNC was delivered at three sites (fundus, anterior, and posterior part) of the myometrium (1 ml at each site). The patient was discharged after 2 h on oral antibiotics, tablet cefixime 200 mg twice a day, for 5 days.
Postprocedure, the patients were started on oral estradiol valerate tablets 2 mg thrice daily for 12 weeks. Medroxyprogesterone 10 mg once a day was given in the last 10 days in patients with ET >6 mm at 3 months of follow-up. If ET <6 mm was found at 3 months of follow-up, the patients were given oral estradiol valerate 2 mg thrice daily for additional 3 months followed by medroxyprogesterone course.
Subjects were followed up at 3, 6, and 9 months and 5 years. At the follow-up, menstrual history and ET by TVS between day 14 and 18 were noted. At 3, 6, and 9 months, all 25 patients followed up. At the end of 5 years, 15 patients followed up. Apart from evaluation of menstrual history and ET, diagnostic hysteroscopy was performed in all the 15 patients (7 with AS and 8 with EA) who followed up at the end of 5 years. IVF treatment was planned for all 25 patients if ET >7 mm on any of the follow-up visits.
Primary outcome measure was endometrial regeneration assessed by resumption of menses or improvement in duration/intensity of menses and improvement in ET and hysteroscopy findings. Secondary outcome measure was functioning of regenerated endometrium assessed by successful pregnancy rate.
Statistical analysis
Data analysis was carried out using statistical software STATA version 12.0 (StataCorp, 2011, Stata Statistical Software: Release 12 College Station, TX, StataCorp LP). Normality assumption of continuous variables was tested using Kolmogorov–Smirnov test. For continuous variables of normally distributed, descriptive statistics such as mean, standard deviation, and range values were calculated. Correlation between MNC count and increase in ET was tested using Spearman's coefficient of rank correlation. Relative change in ET due to treatment from baseline to follow-up times at 3, 6, and 9 months and 5 years was tested using paired t-test. For all the statistical tests, P < 0.05 was considered for statistical significance.
RESULTS
Twenty-five patients were included in the study (12 patients with AS and 13 patients with EA). All the patients with AS had undergone hysteroscopic adhesiolysis once or twice followed by oral estrogen treatment for 2–3 months. The mean duration (months) between hysteroscopic adhesiolysis and stem cell transfer was 27.64 ± 6.26. All patients with EA (ET < 5 mm) had received oral estrogen (1–2 months) in the past and had failed to respond.
The mean age (years) of the study patients was 29.6 ± 4.1 [Table 1]. Sixteen patients presented with primary infertility and the rest with secondary infertility. Among all study patients, 13 patients presented with scanty menses, seven patients had amenorrhea, and five patients did not have any menstrual abnormality. Of the 12 patients with AS, nine had history genital TB, two had history of dilatation and curettage (D and C), and one had history of cesarean section (CS) followed by postpartum sepsis. Of the 13 patients with atrophic endometrium, six had a history of genital TB, two had a history of D and C, two had previous CS, and in three patients, no cause was found. Genital TB was diagnosed on endometrial aspirate by the presence of epithelioid granulomas in 12 patients and positive acid-fast bacilli culture in three patients. The mean MNC count was 65.3 × 10[6] ± 37.2 and ranged from 19 × 10[6] to 200 × 10[6].
Table 1.
Baseline characteristics
| Baseline characteristics | Mean±SD (%) |
|---|---|
| Age (years) | 29.6±4.1 |
| BMI (kg/m2) | 23.7±2.6 |
| FSH (IU/ml) | 6.5±2.3 |
| LH (IU/ml) | 5.1±2.8 |
| Genital TB (%) | 60 |
BMI=Body mass index, FSH=Follicle-stimulating hormone, LH=Luteinizing hormone, TB=Tuberculosis, SD=Standard deviation
Six out of the seven amenorrheic patients resumed menses 3 months poststem cell instillation and continued to menstruate till 9 months. At the end of 5 years, three of these patients who followed up continued to menstruate. Among the 20 patients with scanty menses or amenorrhea, the duration of menses (days) significantly increased from 1.3 ± 0.9 prestem cell instillation to 2.9 ± 0.9 at 3 months (P < 0.001). At 6 months, the duration increased to 3.2 ± 1.0 days (P = 0.203) and 3.3 ± 1.0 days at the end of 9 months (P = 0.163). Menstrual volume assessed by the number of pads used per menstrual cycle also significantly increased from 1.3 ± 0.9 pads prestem cell therapy to 4.4 ± 2.0 pads at 3 months (P < 0.001). At 6 and 9 months, pad usage increased to 4.9 ± 2.1 (P = 0.336) and 5.0 ± 2.1 (P = 0.330).
Mean prestem cell transfer ET (mm) was 3.3 ± 1.0. At the end of 3 months of stem cell treatment, it significantly increased to 5.1 ± 1.9 (P = 0.001). At the end of 6 months (5.6 ± 1.5; P = 0.164) and 9 months (6.1 ± 1.7; P = 0.135), there was a slight improvement in the mean ET, but the difference was not statistically significant. The changes in the mean ET of the 15 patients who followed at the end of 5 years also showed significant increase at end of 3 months but no significant improvement at 6 months, 9 months, and 5 years [Figure 1]. AS and EA subgroups when analyzed separately also showed a similar trend in increase in mean ET [Table 2]. Serial ultrasound images of ET of a patient with AS over the 5-year follow-up period are shown in Figure 2. No correlation was found between MNC count and increase in ET (P = 0.894).
Figure 1.

Change in mean endometrial thickness over the 5 years of follow-up period
Table 2.
Subgroup changes in endometrial thickness (mean±standard deviation) at 5-year follow-up
| Condition | Pre-ET (mm) | ET at 3 months (mm) | P | ET at 6 months (mm) | P | ET at 9 months (mm) | P | ET at 5 years (mm) | P |
|---|---|---|---|---|---|---|---|---|---|
| AS (n=7) | 2.6±0.9 | 4.2±1.3 | 0.001 | 4.6±1.5 | 0.631 | 4.8±1.5 | 0.766 | 5.2±1.3 | 0.655 |
| Endometrial atrophy (n=8) | 3.6±0.7 | 5.9±2.6 | 0.043 | 6.5±1.0 | 0.409 | 7.1±0.8 | 0.051 | 7.3±0.8 | 0.515 |
ET=Endometrial thickness, AS=Asherman’s syndrome
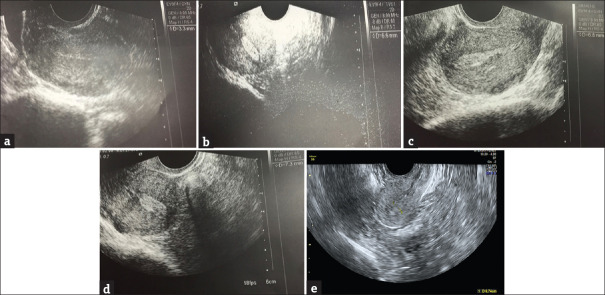
Figure 2.
Ultrasound images of endometrial thickness of a case in the follow-up period. (a) Prestem cell endometrial thickness – 3.3 mm, (b) 3-month endometrial thickness – 6.6 mm, (c) 6-month endometrial thickness – 6.8 mm, (d) 9-month endometrial thickness – 7.3 mm, (e) 5-year endometrial thickness – 7.4 mm
Six out of the seven patients with AS who followed up at the end of 5 years were found to have an improved uterine cavity on diagnostic hysteroscopy. One patient with Grade III adhesions improved to Grade I adhesions [Figure 3], two patients with Grade III adhesions improved to Grade II adhesions, one patient with Grade II adhesions improved to Grade I adhesions, two patients with Grade I adhesions previously were found to have a normal uterine cavity, but one patient with Grade IV adhesions showed no improvement.
Figure 3.
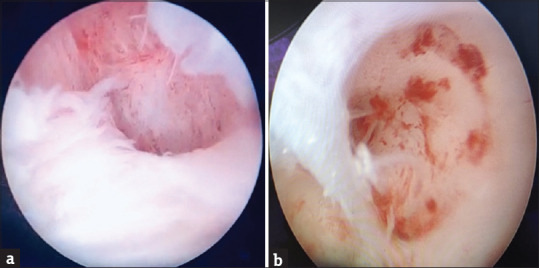
Hysteroscopic images of a case with successful pregnancy outcome pre- and post-stem cell transfer. (a) Prestem cell transfer (Grade III adhesions). (b) Poststem cell transfer (5 years) (Grade I adhesions)
Eleven of 25 patients attained ET >7 mm during the follow-up period and received IVF treatment. Rest were not offered IVF in view of suboptimal ET. Of the 15 patients who followed up at 5 years, two patients spontaneously conceived 3 years poststem cell implantation and one patient conceived 4 years poststem cell implantation by IVF. All 3 had a successful pregnancy outcome (20%). one patient had an ectopic pregnancy (IVF conception) for which she underwent salpingectomy. No adverse effects were noted in any of the patients in the immediate poststem instillation period or during follow-up. Patient details of all Asherman's cases and EA cases are shown in Tables 3 and 4, respectively.
Table 3.
Patient details-Asherman’s syndrome
| Number | Baseline characteristics |
Prestem cell transfer characteristics |
Primary outcome |
Secondary outcome Pregnancy |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Cause | Preoperative menstrual history | Maximum preoperative ET (mm) | Hysteroscopy |
Postoperative menstrual history | Maximum postoperative ET (mm) | |||
| Preoperative | Postoperative (5 years) | ||||||||
| 1 | 29 | CS | Amenorrhea | 1.2 | AS III | AS II | Scanty | 6.7 (6 months) | No |
| 2 | 34 | D and C | Amenorrhea | 2.2 | AS III | AS I | Normal | 6.8 (9 months) | 3 years, spontaneous conception, 38 weeks, VD, 2750 g |
| 3 | 30 | TB | Amenorrhea | 1.7 | AS III | AS II | Scanty | 6.4 (6 months) | No |
| 4 | 23 | TB | Scanty | 3.3 | AS I | Normal | Normal | 7.4 (5 years) | No |
| 5 | 30 | TB | Scanty | 3.8 | AS I | Normal | Normal | 6.9 (6 months) | No |
| 6 | 25 | TB | Amenorrhea | 2.8 | AS IV | AS IV | Scanty | 4.4 (5 years) | No |
| 7 | 24 | D and C | Amenorrhea | 2.1 | AS II | AS I | Scanty | 5 (9 months) | 3 years, Spontaneous conception, 35 weeks, CS, 2250 g |
| 8 | 32 | TB | Scanty | 4.6 | AS I | - | Normal at 6 months, scanty at 9 months | 8.3 (9 months) | No |
| 9 | 29 | TB | Scanty | 3.7 | AS I | Normal at 3 months, scanty at 6 and 9 months | 7.1 (6 months) | No | |
| 10 | 30 | TB | Scanty | 3.2 | AS II | Scanty | 5.9 (9 months) | No | |
| 11 | 24 | TB | Amenorrhea | 2 | AS II | Amenorrhea | 6.4 (6 months) | No | |
| 12 | 30 | TB | Amenorrhea | 3 | AS IV | Normal at 3 months, scanty at 6, 9 months | 6.2 (9 months) | No | |
TB=Tuberculosis, ET=Endometrial thickness, D and C=Dilation and curettage, AS=Asherman’s syndrome, VD=Vaginal delivery, CS=Cesarean section
Table 4.
Patient details-endometrial atrophy
| Number | Baseline characteristics |
Prestem cell transfer characteristics |
Primary outcome |
Secondary outcome Pregnancy |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Cause | Preoperative menstrual history | Maximum preoperative ET (mm) | Hysteroscopy |
Postoperative menstrual history | Maximum postoperative ET (mm) | |||
| Preoperative | Postoperative (5 years) | ||||||||
| 1 | 27 | TB | Scanty | 3.6 | Pale | Scanty | 7.1 (9 months) | 2 years, IVF, ectopic, salpingectomy | |
| 2 | 30 | D and C | Scanty | 3.4 | Pale | Normal | Scanty at 3 and 6 months, normal at 9 months | 7.8 (9 months) | 4 years, IVF conception, 34 weeks, CS, 1800 g |
| 3 | 28 | Unknown | Scanty | 3.3 | Pale | Normal | Normal | 6.6 (9 months) | No |
| 4 | 35 | D and C | Scanty | 3.5 | Pale | Normal | Normal | 7 (5 years) | No |
| 5 | 25 | TB | Scanty | 4.1 | Pale | Normal | Normal | 9.3 (5 years) | No |
| 6 | 24 | unknown | Scanty | 4.9 | Pale | Pale | Normal at 3 months, scanty at 6 and 9 months | 8.8 (9 months) | No |
| 7 | 33 | TB | Scanty | 3.5 | Normal | Normal | Scanty at 3 months, normal at 6 and 9 months | 12 (3 months) | No |
| 8 | 38 | TB | Normal | 2.5 | Normal | Normal | Normal | 8.2 (5 years) | No |
| 9 | 30 | CS | Normal | 4.2 | Normal | Normal | Normal | 6.4 (5 years) | No |
| 10 | 37 | Unknown | Normal | 4 | Pale | Normal | 6.3 (6 months) | No | |
| 11 | 34 | CS | Normal | 4 | Pale | Normal | 9.1 (6 months) | No | |
| 12 | 33 | TB | Normal | 4.5 | Normal | Normal | 6.5 (9 months) | No | |
| 13 | 28 | TB | Scanty | 4.7 | Pale | Normal at 3 months, scanty at 6 and 9 months | 5.6 (3 months) | No | |
CS=Cesarean section, IVF=In vitro fertilization, TB=Tuberculosis, ET=Endometrial thickness
DISCUSSION
In our study, 25 infertile patients with refractory AS and EA underwent autologous BM-derived intrauterine stem cell instillation and were followed at 0, 3, 6, and 9 months and 5 years. The conclusion was restoration of menstruation in majority of amenorrheic patients (6 out of 7) and improvement in menstrual duration and intensity. There was a significant increase in ET 3 months' poststem cell instillation but no further significant improvement up to 5 years. Three patients had a successful pregnancy outcome in the 5-year follow-up period.
In 2004, Taylor was the first to prove that BM stem-derived stem cells play a role in the regeneration of endometrium by detecting donor-derived endometrial cells in the endometrial biopsy samples of BM recipients.[15] In 2011, Nagori et al. described a case of AS treated with autologous BM stem cells for endometrial regeneration that resulted in conception after IVF-ET.[8] Over the recent years, various studies on murine models have shown the role of BM stem cells in endometrial regeneration.[16,17,18,19] Before the present study, our unit conducted a pilot study in 2012–2013 (published in 2014) in which six cases of refractory AS with amenorrhea and infertility received BM-derived stem cell treatment. In 9-month follow-up period, significant improvement in menstruation and ET was seen.[10] The largest study to date on this novel therapy has been published by Santamaria et al. in 2016. It was a pilot study in which 16 cases with refractory AS (11) or EA (5) underwent autologous BM stem cell treatment. Santamaria et al. followed up the study subjects at 3 and 6 months to assess menstrual history, ET, adhesion score, and neoangiogenesis. Fifteen out of 16 patients resumed menses though duration and intensity of menstruation decreased progressively 6 months poststem cell treatment. Hysteroscopic visualization of the uterine cavity, ET, and neoangiogenesis observed through immunofluorescence markedly improved. Two term live births and two ongoing pregnancies were achieved.[11] In our study, maximum improvement in menstruation and ET was seen at 3 months of follow-up. In our subsequent long-term follow-up, the effect plateaued but sustained itself. Three successful pregnancies were achieved after a long period of poststem cell treatment (3–4 years).
Santamaria et al. found a history of D and C as the most common cause of AS.[11] In our study, genital TB was the most common etiology of AS and EA. However, interestingly, all three pregnancies were in the group that underwent D and C in the past and none in the other groups.
Nagori et al. instilled stem cells in the endometrial cavity using ET cannula under transabdominal ultrasound guidance. Endometrial curettage was required before procedure to allow the instilled stem cells to reach basal layer of endometrium.[8] In the study by Santamaria et al., radiologist performed femoral artery catherization for injecting stem cells into the uterine artery.[11] In our study stem cells, no endometrial curettage was done before procedure, and stem cells were implanted directly into the subendometrial zone under ultrasound guidance using the ovum pick-up needle (Cook No. 17). Our method was easy to perform, not requiring radiologist intervention, and yet it ensured that stem cells are instilled close to the basal layer of endometrium.
Santamaria et al. identified and exclusively transferred CD133+ BM stem cells.[11] CD133+ is a proven endothelial progenitor cell marker with a known safety profile.[20] Nagori et al. instilled endometrial angiogenic stem cell markers CD9, CD44, and CD90+ stem cells.[8] CD34+ is also an endothelial progenitor marker which helps in angiogenesis and tissue repair.[21,22] In our pilot study, CD34+ stem cells were quantified; however, all mononuclear stem cells were injected. No correlation was seen between CD34+ cell count and improvement in ET.[10] Hence, in our present study, BM-derived noncharacterized mononuclear stem cells were injected and CD34+ cell quantification was not performed. Endometrial regeneration in our patients could be due to the incorporated mononuclear stem cells (hemopoietic and nonhemopoietic) by differentiating into endometrial epithelium and stroma or by activating the resident endometrial progenitor cells by providing growth factors, to proliferate and replace the lost cells.
Various stem cell markers have been tried in previously published studies, but currently, there is no clarity on which stem cell marker is most suitable for endometrial regeneration. However, no adverse effects due to uterine stem cell instillation were noted in our study or any of the previously published literature.
Although there was an improvement in amenorrhea and menstrual flow, our primary intention is to achieve a pregnancy. Only one out of the 25 patients could achieve a successful pregnancy through IVF (2 pregnancies were spontaneous conceived). Hence, IVF treatment is not making a dramatic difference in these patients. Significant change in ET has been observed in 6 months of the procedure in all studies so far. This implies that fertility treatment should be offered earlier.
Our study had a follow-up period of 5 years (longest in comparison with published literature) in which menstrual pattern and ET were periodically recorded (3, 6, and 9 months and 5 years). Diagnostic hysteroscopy being an invasive procedure was not performed periodically at 3, 6, or 9 months but was performed at the 5-year follow-up visit. Strengths of our study were use of a simple yet robust method of stem cell instillation and a long follow-up period. Limitations were small sample size (largest among published literature to date) and loss to follow-up (due to a long follow-up period).
CONCLUSION
Intrauterine stem cell treatment is a promising novel approach for refractory cases of AS and EA. Particularly, in today's era, where commercial surrogacy is under the scanner and has been banned in many parts of the world, this treatment is a ray of hope for these patients. This approach at present is in its experimental phase. Future research should aim at larger studies to further strengthen evidence to support its benefit and to find a specific stem cell-based therapy whose benefit can be provided outside clinical trials to the general public.
Financial support and sponsorship
Research grant by All India Institute of Medical Sciences, Delhi, supported the study.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome – One century later. Fertil Steril. 2008;89:759–79. doi: 10.1016/j.fertnstert.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 3.Panayiotides I, Weyers S, Bosteels J, Herendae VB. Intrauterine adhesion (IUA): Has there been progress in understanding and treatment over last 20 years? Gynecol Surg. 2009;6:197–211. [Google Scholar]
- 4.Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: A review of literature. Reprod Biol Endocrinol. 2013;11:118. doi: 10.1186/1477-7827-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valle RF, Sciarra JJ. Intrauterine adhesions: Hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol. 1988;158:1459–70. doi: 10.1016/0002-9378(88)90382-1. [DOI] [PubMed] [Google Scholar]
- 6.Senturk LM, Erel CT. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008;20:221–8. doi: 10.1097/GCO.0b013e328302143c. [DOI] [PubMed] [Google Scholar]
- 7.Mouhayar Y, Sharara F. Modern management of thin lining. Middle East Fertil Soc J. 2017;22:1–2. [Google Scholar]
- 8.Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome. J Hum Reprod Sci. 2011;4:43–8. doi: 10.4103/0974-1208.82360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassena R, Eguizabal C, Heindryckx B, Sermon K, Simon C, van Pelt AM, et al. Stem cells in reproductive medicine: Ready for the patient? Hum Reprod. 2015;30:2014–21. doi: 10.1093/humrep/dev181. [DOI] [PubMed] [Google Scholar]
- 10.Singh N, Mohanty S, Seth T, Shankar M, Bhaskaran S, Dharmendra S. Autologous stem cell transplantation in refractory Asherman's syndrome: A novel cell based therapy. J Hum Reprod Sci. 2014;7:93–8. doi: 10.4103/0974-1208.138864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: A pilot cohort study. Hum Reprod. 2016;31:1087–96. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 12.Wamsteker K. European Society of Hysteroscopy (ESH) Classification of IUA. 1989 [Google Scholar]
- 13.Neagu M, Sicui E, Ordodi V, Paunescu V. Human mesenchymal stem cell as basic tools for tissue engineering: Isolation and culture. Rom J Biophys. 2012;15:29–34. [Google Scholar]
- 14.Gilmore MJ, Prentice HG, Blacklock HA, Janossy G, Hoffbrand AV. A technique for rapid isolation of bone marrow mononuclear cells using Ficoll-Metrizoate and the IBM 2991 blood cell processor. Br J Haematol. 1982;50:619–26. doi: 10.1111/j.1365-2141.1982.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–5. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 16.Alawadhi F, Du H, Cakmak H, Taylor HS. Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One. 2014;9:e96662. doi: 10.1371/journal.pone.0096662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing Z, Qiong Z, Yonggang W, Yanping L. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat. Fertil Steril. 2014;101:587–94. doi: 10.1016/j.fertnstert.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 18.Kilic S, Yuksel B, Pinarli F, Albayrak A, Boztok B, Delibasi T. Effect of stem cell application on Asherman syndrome, an experimental rat model. J Assist Reprod Genet. 2014;31:975–82. doi: 10.1007/s10815-014-0268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J, Zhang Q, Wang Y, Li Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod Sci. 2015;22:181–8. doi: 10.1177/1933719114537715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 21.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): Controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–46. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplan AI. Why are MSCs therapeutic? New data: New insight. J Pathol. 2009;217:318–24. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]