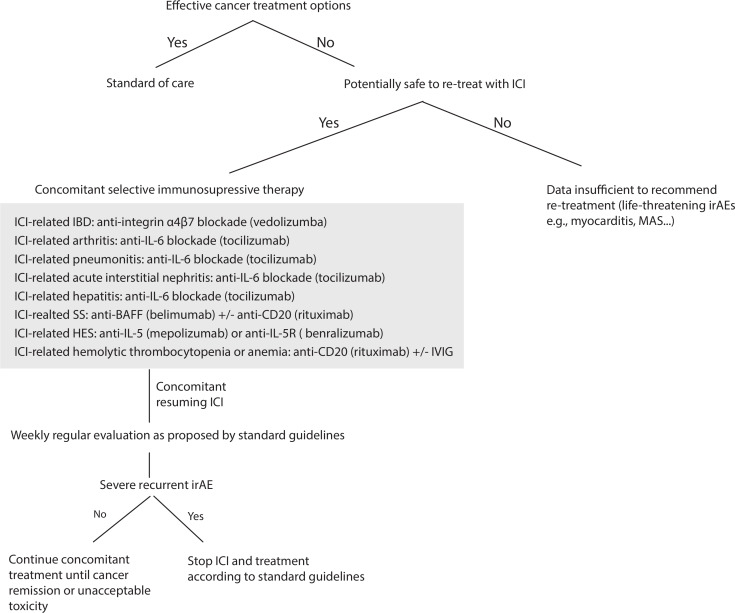
Figure 1.
Flow chart for a suggested secondary prevention for ICI resumption after severe irAEs. For patients with no effective cancer treatment options and if there is no major contraindication related to a life-threatening irAEs, ICI could be resumed concomitantly with a prophylactic selective immunosuppressive therapy (SI). For severe ICI-related colitis, we advocate resuming ICI under VDZ, for severe ICI-related SS under anti-BAFF inhibitor±anti CD20 depletion, for severe ICI-related arthritis irAEs under TCZ, for ICI-related hemolytic anemia and thrombocytopenia irAEs under anti-CD20 depletion±IVIG. For ICI-related HES under anti-IL5(R), ICI-related pneumonitis under TCZ, ICI-related hepatitis under TCZ and ICI-related acute interstitial nephritis under TCZ. If patients experienced new or recurrent high-grade irAEs despite concomitant SI, we advocate the definitive ICI discontinuation and consideration of prompt local expertize for appropriate immunosuppressive treatment. VDZ 300 mg once every 2 months, belimumab (BLM) at 10 mg/kg every 4 weeks, TCZ 162 mg subcutaneously once per week or biweekly; or intravenously at 8 mg/kg once per month, RTX 500 mg to 1000 mg biweekly for 2 x, mepolizumab 300–750 mg monthly and benralizumab at 30 mg once monthly. BAFF, B-cell activating factor; HES, hypereosinophilic syndrome; IBD, inflammatory bowel disease; ICI, immune checkpoint inhibitor; IL-5, interleukin-5; irAEs, immune-related adverse events; IVIG, intravenous immunoglobulin; MAS, macrophage activation syndrome; PMR, polymyalgia rheumatica; RTX, rituximab; SS, Sjögren syndrome; TCZ, tocilizumab; VDZ, vedolizumab.