Figure 5. Macrophage sensors reflect immunological timeframe in two models of acute inflammation and wound healing.
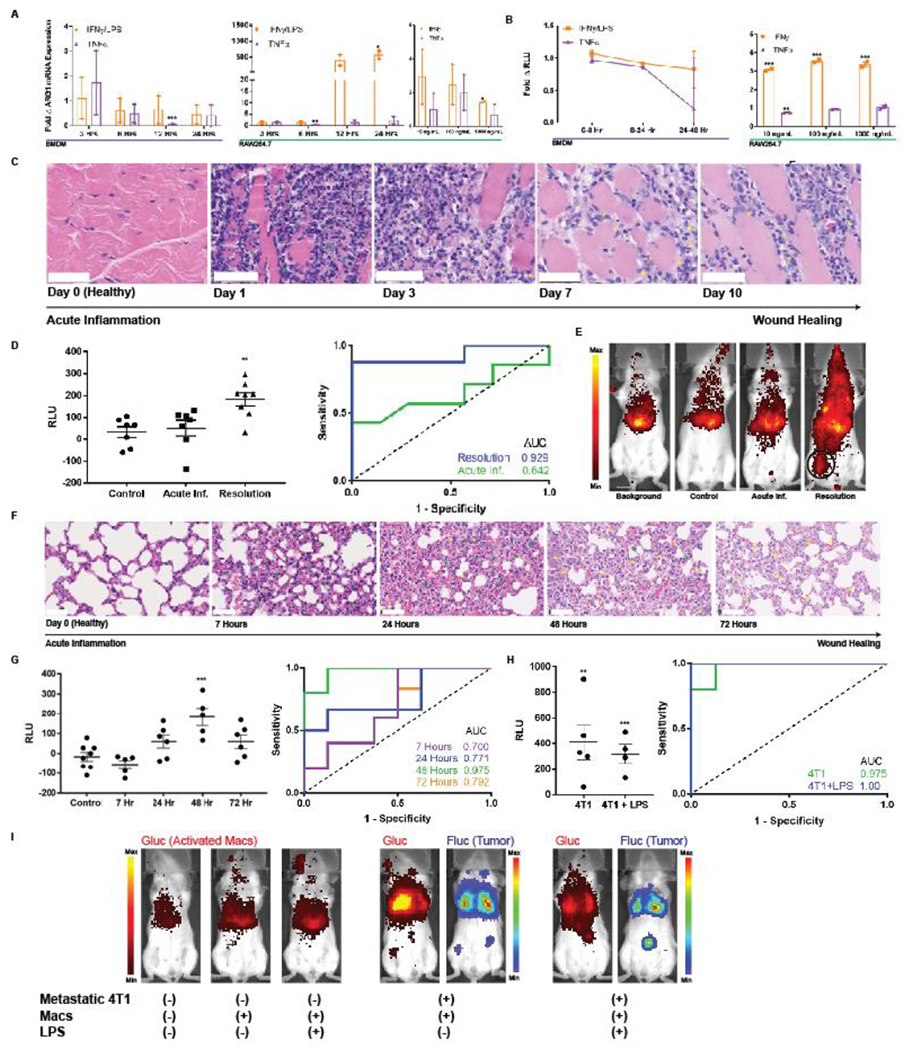
(A) BMDMs and RAW264.7 macrophages exhibit minimal elevations in ARG1 mRNA upon exposure to classic pro-inflammatory cytokines IFNγ and TNFα as quantified by qPCR. BMDM ARG1 levels are similarly not affected by LPS. (B) The engineered pARG1-Gluc expressing macrophage sensors do not result in notably increased Glue levels in culture media upon stimulation with the same pro-inflammatory cytokines. (C) H&E stains of hind leg muscle from days 0-10 post intramuscular injection of turpentine oil exhibit a classical timeline of acute inflammation with a primary neutrophilic (green arrows) response followed by infiltration of macrophages (yellow arrows) in the later stages of inflammation resolution. Scale bars measure 50 pm. (D) Background subtracted plasma Glue levels from the macrophage sensor either on day 1 (n = 6) or day 7 (n = 8) of inflammation (left) revealed no elevation in the acute inflammatory phase (day 1) but significant elevation and macrophage activation in the resolution phase (day 7). This is also reflected by an undiscriminating AUC = 0.643 (95% CI 0.332-0.953, p = 0.371) during acute inflammation but a robust AUC = 0.929 (95% CI 0.783-1.00, p = 0.006) during the wound healing phase. (E) BLI of intracellular Glue from activated macrophages reveals comparable signal from background, non-inflamed mice injected with sensor (control), and acutely-inflamed mice injected with sensor (Acute Inf.). This contrasts with BLI images when macrophage sensor is injected during the resolution phase on day 7 wherein localized activation of macrophages at the site of wound healing (black circle) is clearly visible. (F) H&E stains of lungs following intranasal inoculation with LPS exhibit a similar timeline of acute inflammation with a neutrophilic infiltrate (green arrows) present at 7 hours followed by gradual replacement with macrophages (yellow arrows) as the wound healing process progresses. Wound healing peaks at 48 hours after LPS inoculation and by 72 hours there is some restoration of healthy lung architecture. Scale bars measure 50 μm. (G) Plasma Glue measurements of mice injected with BMDM sensor reflect the acute inflammation and wound healing kinetics peaking at 48 hours with an AUC = 0.975 (95% CI 0.900-1.00, n = 5, p = 0.0054). (H) The BMDM sensor can robustly discriminate metastatic 4T1 tumors both in the absence (AUC = 0.975, 95% CI 0.900–1.00, n = 5, p = 0.0054) and presence (AUC = 1.00, 95% CI 1.00-1.00, n = 4, p = 0.0066) of LPS-induced acute inflammation via plasma Glue measurements as well as (I) via BLI of activated macrophages * indicates statistical significance at p < 0.05, ** indicates statistical significance at p < 0.01, and *** indicates statistical significance at p < 0.001. Error bars depict s.e.m. LPS, lipopolysaccharide; RLU, relative luminescence units; AUC, area under the curve.
