Abstract
Objective
To assess the effect on survival of extent of lymphadenectomy during esophagectomy for patients undergoing multimodality (neoadjuvant) therapy for adenocarcinoma of the esophagus and esophagogastric junction using Worldwide Esophageal Cancer Collaboration data.
Summary Background Data
Prior worldwide data demonstrated that optimum lymphadenectomy during esophagectomy alone for esophageal cancer provides accurate staging and maximum survival. However, for patients undergoing neoadjuvant therapy for locally advanced adenocarcinoma, its value is unclear, leading to wide practice variability.
Methods
A total of 3,859 patients with adenocarcinoma of the esophagus or esophagogastric junction received neoadjuvant therapy. The endpoint was all-cause mortality, reported as gain or loss of lifetime within 10 years. Lifetime predicted for each regional lymph node resected used quantile survival random forest methodology.
Results
Across all post-neoadjuvant ypTNM cancer categories, some degree of lymphadenectomy was associated with longer lifetime, but in a nonlinear fashion. For patients with ypN0 cancers, there was a modest gain in lifetime up to 25 lymph nodes resected and an incremental loss in lifetime as more than 25 were resected. For patients with ypN+ cancers, there was a robust gain in lifetime up to 30 lymph nodes resected and then an incremental loss in lifetime.
Conclusions
Worldwide data for adenocarcinoma of the esophagus and esophagogastric junction demonstrate that lymphadenectomy during esophagectomy is a valuable component of neoadjuvant therapy. Survival is maximized when an optimum range of nodes are resected.
MINI-ABSTRACT
Worldwide data for esophageal adenocarcinoma demonstrate that lymphadenectomy during esophagectomy is a valuable component of multimodality (neoadjuvant) therapy. Survival is longest within an optimum range of resected nodes, but shorter when too few.
INTRODUCTION
Lymphadenectomy has a defined role in managing esophageal and esophagogastric junction cancer in patients undergoing esophagectomy alone.1 Optimum lymphadenectomy provides accurate staging, maximum survival, and can guide therapy. However, its value and extent during esophagectomy as a component of multimodality (neoadjuvant) therapy in treating esophageal adenocarcinoma is debated.2,3 Therefore, purposes of this study were to use Worldwide Esophageal Cancer Collaboration (WECC) data4–9 for neoadjuvant therapy to 1) assess whether lymphadenectomy offers any survival benefit and, if so, 2) determine optimum lymphadenectomy with respect to survival.
METHODS
Patients and Therapies
At 33 WECC institutions (Appendix 1), 13,365 patients with adenocarcinoma of the esophagus or esophagogastric junction underwent esophagectomy, among whom 4,673 had neoadjuvant therapy. Of these, 3,859 patients in 22 institutions had data available for the number of lymph nodes resected and whether lymph nodes were (ypN+) or were not (ypN0) positive for cancer (chemotherapy in 868, radiotherapy in 32, both in 2,934, and unstated in 25). Patients having both neoadjuvant therapy and post-esophagectomy adjuvant therapy were not included (Tables 1 and 2). Approach to esophagectomy was minimally invasive (total or hybrid) in 678 (19% of 3,613 in whom approach was known), hiatal in 642 (18%), thoracotomy in 1,852 (51%), thoracoabdominal in 441 (12%), and unstated in 246.
Table 1.
Baseline characteristics of patients with adenocarcinoma of the esophagus
| Characteristics | Overall (n=3,859) |
ypN0 (n=2,078) |
ypN+ (n=1,781) |
|||
|---|---|---|---|---|---|---|
| na | No. (%) or Mean ± SD | na | No. (%) or Mean ± SD | na | No. (%) or Mean ± SD | |
| Demographics | ||||||
| Age (y) | 3,689 | 61 ± 9.9 | 1,968 | 62 ± 9.7 | 1,721 | 61 ± 10 |
| Female | 3,859 | 453 (12) | 2,078 | 248 (12) | 1,781 | 205 (12) |
| Body mass index (kg/m2) | 2,610 | 28 ± 5.2 | 1,551 | 28 ± 5.2 | 1,059 | 27 ± 5.2 |
| Weight loss (kg) | 1,341 | 4.8 ± 8.5 | 713 | 4.4 ± 7.0 | 628 | 5.3 ± 9.9 |
| Comorbidities | ||||||
| ECOG performance status | 1,403 | 882 | 521 | |||
| 0 | 559 (40) | 368 (42) | 191 (37) | |||
| 1 | 802 (57) | 485 (55) | 317 (61) | |||
| 2 | 34 (2.4) | 23 (2.6) | 11 (2.1) | |||
| 3 | 8 (0.57) | 6 (0.70) | 2 (0.40) | |||
| 4 | 0 (0) | 0 (0) | 0 (0) | |||
| Diabetes mellitus | 3,643 | 483 (13) | 1,974 | 267 (14) | 1,669 | 216 (13) |
| Insulin-dependent | 3,501 | 53 (1.5) | 1,887 | 30 (1.6) | 1,614 | 23 (1.4) |
| Non–insulin-dependent | 3,501 | 288 (8.2) | 1,887 | 150 (7.9) | 1,614 | 138 (8.6) |
| Coronary artery disease | 2,639 | 379 (14) | 1,404 | 221 (16) | 1,235 | 158 (13) |
| Arrhythmia | 1,665 | 27 (1.6) | 806 | 13 (1.6) | 859 | 14 (1.6) |
| Hypertension | 2,344 | 670 (29) | 1,172 | 371 (32) | 1,172 | 299 (26) |
| Peripheral arterial disease | 2,692 | 72 (2.7) | 1,410 | 42 (3.0) | 1,282 | 30 (2.3) |
| Smoker | 3,011 | 2,034 (68) | 1,677 | 1,195 (71) | 1,334 | 839 (63) |
| Past | 2,331 | 935 (40) | 1,258 | 535 (43) | 1,073 | 400 (37) |
| Current | 2,331 | 419 (18) | 1,258 | 241 (19) | 1,073 | 178 (17) |
| FEV1 (% of predicted) | 1,946 | 96 ± 19 | 1,155 | 95 ± 19 | 791 | 97 ± 19 |
| FVC (% of predicted) | 1,118 | 100 ± 17 | 643 | 99 ± 17 | 475 | 101 ± 18 |
| Creatinine (mg/dL) | 399 | 76 ± 21 | 239 | 77 ± 21 | 160 | 75 ± 22 |
| Bilirubin (mg/dL) | 163 | 10 ± 7.9 | 84 | 10 ± 5.6 | 79 | 10 ± 9.8 |
Patients with data available.
15th/50th/85th percentiles.
Key: ECOG, Eastern Cooperative Oncology Group; FEV1 (%), forced expiratory volume in 1 second (percent of predicted); FVC (%), forced vital capacity (percent of predicted); SD, standard deviation.
Table 2.
Clinical (c) and post-neoadjuvant therapy pathologic (yp) cancer characteristics
| Characteristic | Clinical |
Pathologic |
||
|---|---|---|---|---|
| na | No. (%) | na | No. (%) | |
| T | 3,605 | 3,610 | ||
| T0 | 9 (0.25) | 606 (17) | ||
| Tis | 5 (0.14) | 12 (0.33) | ||
| T1 | 122 (3.4) | 555 (15) | ||
| T2 | 682 (19) | 706 (20) | ||
| T3 | 2,674 (74) | 1,656 (46) | ||
| T4 | 113 (3.1) | 75 (2.1) | ||
| TX | 254 | 249 | ||
| N | 3,709 | 3,859 | ||
| N0 | 1,308 (35) | 2,078 (54) | ||
| N+ | 2,401 (65) | 1,781 (46) | ||
| N1 | 75 | 55 (73) | 1,779 | 914 (51) |
| N2 | 75 | 18 (24) | 1,779 | 534 (30) |
| N3 | 75 | 2 (2.7) | 1,779 | 331 (19) |
| NX | 150 | 2 | ||
| M | 3,859 | 3,859 | ||
| M0 | 3,664 (95) | 3,664 (95) | ||
| M1 | 195 (5.1) | 195 (5.1) | ||
| Gradee | 1,481 | 3,443 | ||
| G1 | 40 (2.7) | 695 (20) | ||
| G2 | 700 (47) | 1,212 (35) | ||
| G3 | 741 (50) | 1,536 (45) | ||
| G4 | 0 (0) | 0 (0) | ||
| GX | 2,378 | 416 | ||
| Location | 3,646 | 3,646 | ||
| Upper | 22 (0.6) | 22 (0.6) | ||
| Middle | 121 (3.3) | 121 (3.3) | ||
| Lower | 3,503 (96) | 3,503 (96) | ||
| LocationX | 213 | 213 | ||
| Resection | 3,859 | |||
| R0 | — | 3,404 (88) | ||
| R1 | — | 299 (7.7) | ||
| R2 | — | 156 (4.0) | ||
Data
This study used 34 variables from previous analyses of WECC data,4,5,9 with site and continent excluded to contain dimensionality of data and reduce confounding with treatment (Supplemental Digital Content [SDC] Appendix 1: Variables Used in Random Forest Analysis). WECC data were obtained after local ethics-board approval of databases, and data-use agreements were executed with Cleveland Clinic. Data were requested in completely de-identified format (Health Insurance Portability and Accountability Act research standards) for a set of required variables with standard definitions. Variables included demographics, comorbidities, cancer characteristics, cancer treatment, and time-related mortality (see SDC Appendix 1). The Case Cancer Institutional Review Board of Case Western Reserve University and Cleveland Clinic Institutional Review Board approved the entire project and use of these data for research, with patient consent waived.
Endpoint
The endpoint was all-cause mortality from first management decision, induction therapy in this instance. Median potential follow-up10 was 7.1 years (25% >11.5 years, 10% >14.9 years) if there were no deaths. However, considering deaths in this elderly population with a rapidly lethal cancer, 50% of patients were followed more than 1.5 years, with 25% followed more than 3.1 years and 10% more than 5.4 years.
Data Analysis
Analytic Strategy
Primary objectives of the analyses were to determine if lymphadenectomy during esophagectomy offers a survival benefit and, if so, to identify the number of resected lymph nodes predicted to maximize survival. This was accomplished in 3 steps:
Plausible Extents of Lymphadenectomy. We identified from characteristics of patients and their cancers a plausible extent of lymphadenectomy using quantile random forests regression11,12 (SDC Appendix 2: Method for Identifying Plausible Extents of Lymphadenectomy; and SDC Figure 1: Example of how range of plausible lymphadenectomy was determined for 2 patients). For ypN+ cancers, the minimum plausible number of nodes resected must be 1. We truncated the number of nodes resected at 50 (Figure 1).
Survival Analysis for Each Patient. A survival analysis was performed that incorporated interactions of all variables with number of lymph nodes actually resected using an extension of random survival forests13 (SDC Appendix 3: Method for Survival Analysis Using Random Survival Forests, Virtual Twin, with Interactions14). From this analysis, a survival curve for each patient was predicted for actual number of lymph nodes resected. Then a sequence of survival curves for alternative extents of lymphadenectomy for that patient (“what ifs”) was obtained using the same patient and cancer characteristics, but substituting these alternative extents of lymphadenectomy for actual number resected. This generated for each patient a maximum of 51 survival curves, one for each extent of lymphadenectomy for which it was plausible.
Optimum Lymphadenectomy. Length of life for each “what if” number of resected lymph nodes was estimated by restricted mean survival time (“lifetime”),15–17 the area beneath a survival curve from beginning of treatment (induction therapy) to a specified time point. For this study, we chose 10 years as that specific time point and calculated for each patient all plausible lifetimes, expressing lifetime in months. We defined optimum lymphadenectomy as the plausible number of lymph nodes resected that yielded maximum lifetime. These lifetime values were summarized separately for patients post-induction therapy who did not or did have regional lymph nodes positive for cancer, ypN0, and ypN+.
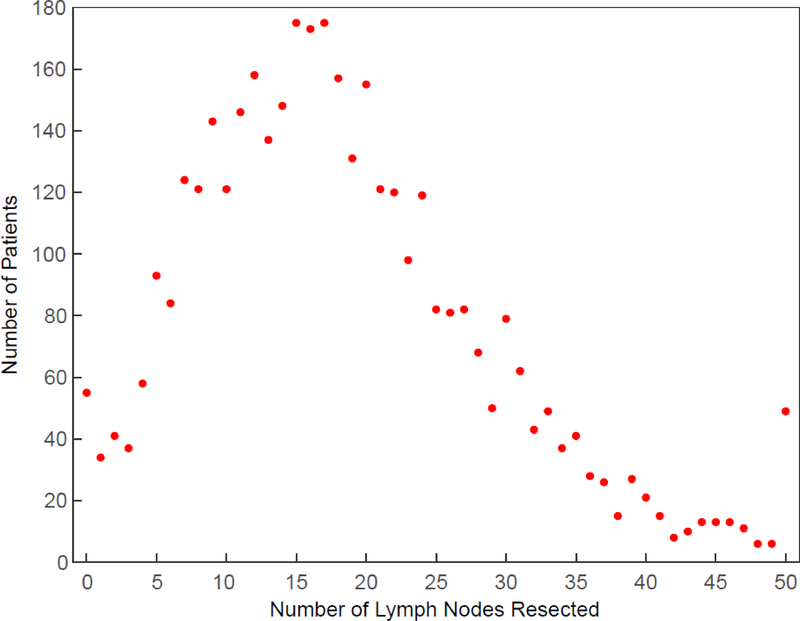
Figure 1:
Extent of lymphadenectomy. Each dot depicts number of patients with that number of lymph nodes resected. Last dot represents 50 or more nodes resected. Of the 3,859 patients, 55 (1.4%) had 0 nodes resected; 735 (19%) 1–9; 1,521 (39%) 10–19; 976 (25%) 20–29; 523 (14%) 30–49; and 49 (1.3%) 50 or more.
Missing Data
Missing data for covariates were imputed using “on the fly” random forest imputation18 implemented in the open source randomForestSRC R package under default settings.13
RESULTS
Extent of Lymphadenectomy
Number of lymph nodes resected during esophagectomy for adenocarcinoma of the esophagus peaked between 15 and 17. Forty-nine patients had 50 or more nodes resected and 55 had 0 nodes resected. Among 16 institutions of the 22 that reported on 20 or more patients, the percentage of patients with 30 or more lymph nodes resected varied from 0% to 35%, median 12% (SDC Table 1, showing institutional volume and extent of lymphadenectomy). Extensive lymphadenectomy of 30 or more nodes varied according to surgical approach to esophagectomy: 4.2% (27/642 patients) during transhiatal esophagectomy, 14% (64/441 patients) during thoracoabdominal esophagectomy, 16% (106/678 patients) during minimally invasive esophagectomy, and 19% (357/1,852 patients) during esophagectomy via thoracotomy, with transhiatal esophagectomy being significantly fewer than other approaches (P[Bonferoni adjusted] <.0001).
Value of Lymphadenectomy
Across all ypT and ypN classifications, there was a predicted survival benefit of at least some degree of lymphadenectomy greater than 0 nodes resected (Figure 2), although in a nonlinear parabolic fashion (Figures 3 and 4, and see SDC Figure 2, which shows predicted lifetime across all ypT categories and 4 groups of number of lymph nodes resected for ypN0M0 cancers; and SDC Figure 3, which shows the same data for ypN+ cancers). Increasing number of lymph nodes resected was associated with a gain in lifetime up to a point, after which a decrease in lifetime was observed for both ypN0 and ypN+ cancers (see Figure 2). Overall survival was 98.2% and 98.1% at 30 days for both ypN0 and ypN+ patient cohorts, respectively, highest for the 55 with no nodes resected (SDC Table 2, showing 30-day mortality according to extent of lymphadenectomy).
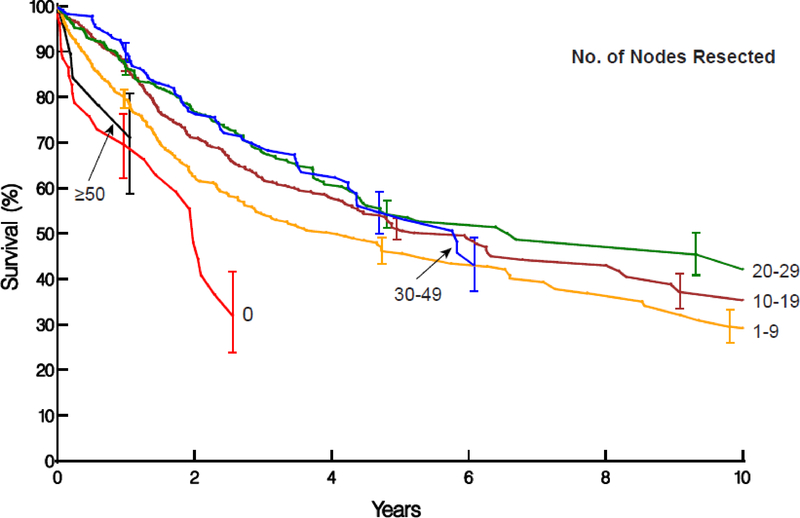
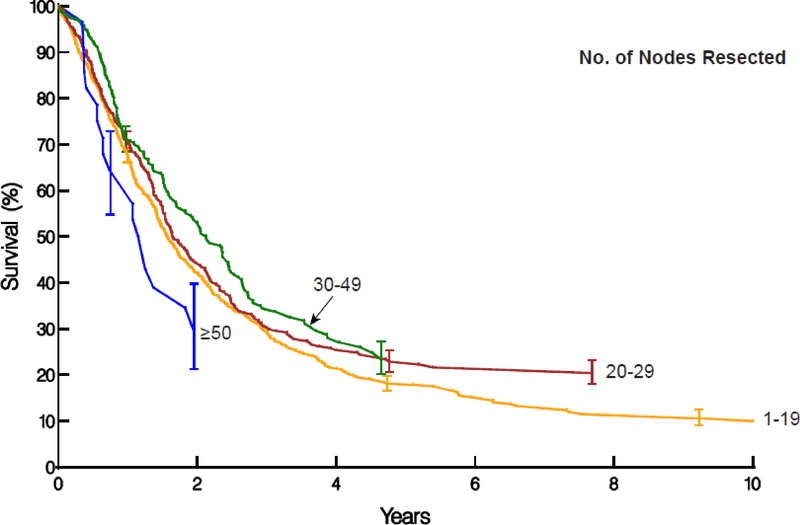
Figure 2:
Non–risk-adjusted survival stratified by number of lymph nodes resected. Symbols represent deaths and vertical line asymmetric confidence intervals equivalent to ±1 standard error.
A, ypN0M0 cancers with survival stratified by 0, 1–9, 10–19, 20–29, 30–49, and ≥50 nodes resected. These curves show the value of >0 nodes resected, increasing survival until 20 to 29 are resected, and decreasing survival for more extensive lymphadenectomy.
B, ypN+ cancers with survival stratified by 1–19, 20–29, 30–49, and ≥50 nodes resected. Survival is lowest when 1–19 nodes are resected, somewhat higher when 20–29 nodes are resected, but somewhat lower when more nodes are resected.
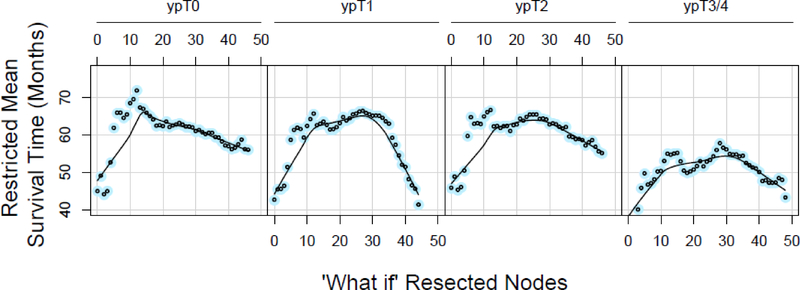
Figure 3:
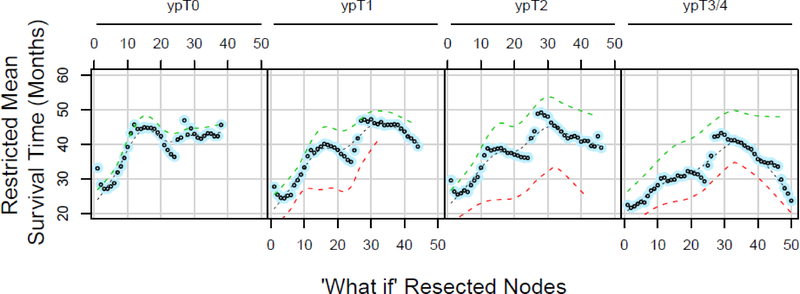
Restricted mean survival time in months for patients with ypN0M0 cancers who had 18 to 25 regional lymph nodes resected. Along the horizontal axis is potential “what if” number of resected nodes, and panels represent ypT categories with ypT3 and ypT4 combined. Each dot represents a minimum of 10 patients. Patients with ypT3 and ypT4 cancers have been combined. These curves are panels I-L of Supplemental Digital Content Figure 2.
Figure 4:
Restricted mean survival time (RMST) in months for patients with ypN+ cancers who had 22–29 regional lymph nodes actually resected. Along the horizontal axis is potential “what if” number of lymph nodes, and panels represent ypT categories, with ypT3 and ypT4 combined. Each dot represents a minimum of 10 patients. Black dashed line is a loess fit to data, green dashed lines show RMST loess fits for patients with 1 or 2 positive lymph nodes, and red dashed lines show loess fits for patients with 3 or more positive nodes. These curves are panels M-P in Supplemental Digital Content Figure 3.
Lymphadenectomy in ypN0M0 Cancers
For patients with ypN0 cancers, there was a predicted gain up to 20 months in potential lifetime associated with up to 25 lymph nodes resected. This is illustrated in Figure 3 and SDC Figure 2, panels M-P, from which Figure 3 was extracted, for ypT0, ypT1, ypT2, and ypT3/4 patients who actually had between 18 and 25 regional lymph nodes resected. However, with a potentially larger number of nodes resected, particularly more than 30, potential lifetime for these “what if” scenarios decreased up to 30 months (see SDC Figure 2, which illustrates this pattern of loss of lifetime).
This pattern was least pronounced among patients with ypT0N0 cancers (complete response) across all ranges of actual lymph nodes resected (see SDC Figure 2, panels A, E, I, M, and Q, which demonstrate this). For patients with residual advanced adenocarcinoma (ypT3/4), the relationship was similarly blunted (see SDC Figure 2, panels D, H, L, P and T, which illustrate this). The peaking parabolic pattern was most pronounced among ypT1N0 and ypT2N0 cancers.
Lymphadenectomy in ypN+M0 Cancers
For patients with ypN+ cancers, there was a substantial predicted gain in lifetime associated with about 30 lymph nodes resected, often 20 more months. This is illustrated in Figure 4 and SDC Figure 3, panels Q-T, from which Figure 4 was extracted for ypT0, ypT1, ypT2, and ypT3/4 patients who actually had 22 to 29 lymph nodes resected. However, with a potentially larger number of nodes resected beyond 30 (see SDC Figure 3, panels Q-T, which illustrate this pattern), potential lifetime for these “what if” cancers decreased.
The decrement in lifetime was blunted in patients with ypT0N+ cancers but robust in higher T categories (see SDC Figure 3, panels A, E, I, M, and Q and panels D, H, L, P, and T). The pattern of incremental increase in lifetime up to a point, followed by a decrement, held true for patients with 1–2 positive lymph nodes as well as for those with 3 or more positive nodes (see SDC Figure 3, green and red dashed lines), although lifetime was substantially shortened when 3 or more nodes were positive.
DISCUSSION
Principal Findings
Based on worldwide data for adenocarcinoma of the esophagus and esophagogastric junction across all post-induction (ypTNM) cancer categories, some degree of lymphadenectomy was found to be associated with longer lifetime, but in a nonlinear parabolic fashion. Increasing number of lymph nodes resected was associated with a gain in lifetime up to a point, after which there was progressive loss in lifetime for both ypTN0 and ypTN+ cancers. For patients with ypN0 cancers, an incremental potential gain in lifetime was predicted for up to 25 lymph nodes resected, and an incremental decrease when a more extensive lymphadenectomy was performed, most pronounced in ypT1–2. For patients with ypN+ cancers, a similar gain in lifetime was predicted for up to about 30 nodes resected, followed by an incremental decrease in lifetime. Given the low perioperative mortality, these findings are not explained by early adverse events. Nor was this finding due to results at a small number of institutions, as most institutions performed extensive lymphadenectomy in a reasonable number of patients.
Value of Lymphadenectomy in Neoadjuvant Therapy
The role of lymphadenectomy in treatment-naïve patients undergoing esophagectomy alone is not in debate, but the role of lymphadenectomy after induction therapy is largely unknown, with a paucity of data that is contradictory. Using the National Cancer Database (NCDB), Samson and colleagues2 evaluated 18,777 patients undergoing esophagectomy and found that lymphadenectomy was limited (<15 lymph nodes resected) in almost 63% of patients having lymphadenectomy. Among those undergoing esophagectomy after induction therapy, patients who had 15 lymph nodes or more resected had a survival benefit compared with those who had fewer nodes resected. In the same year, Giugliano and colleagues3 noted that among 174 patients at their institution undergoing esophagectomy after induction chemoradiotherapy, resection of less than 15 lymph nodes did not affect survival. Both studies used specific lymph node resection cutoff values (greater or less than 15 lymph nodes), with some analyses assessing the hazard ratio in 5-lymph-node increments.
Along this line, an analysis of patients in the CROSS trial found no prognostic effect of extent of lymphadenectomy after induction therapy.19 This finding may be due to the relatively small number of patients (159 vs 3,859 patients) compared with our study. More plausible, based on the parabolic relationship of risk-adjusted survival to extent of lymphadenectomy we have found, is that the assumed linear relationship of survival to number of nodes resected in that analysis was incorrect (model misspecification). It is known that a parabolic relationship, particularly a symmetrical one, will show no linear effect. In contrast to this method, we examined the effect of number of lymph nodes resected as a discrete whole-number variable using nonparametric machine learning without any assumption of the shape of the relationship, examining the effect on survival of each number of nodes resected based on both the actual number of nodes resected and on counterfactual “what if” scenarios for number of nodes resected in an “individual treatment effect” causal inference framework. With this we were able to quantify the survival benefit of lymphadenectomy and describe the shape of the relationship of survival to extent of lymphadenectomy. Use of nonparametric machine learning for the present analysis and those leading to the staging recommendations for both 7th and 8th editions of the AJCC/UICC cancer staging manuals, was in recognition of nonlinear relationships of survival to TNM and non-anatomic cancer characteristics along with strong interactions that are difficult to tease out with traditional statistical methods.20–22
Optimum Lymphadenectomy during Neoadjuvant Therapy
We also report both an incremental survival benefit of more resected nodes and a survival decrement when lymphadenectomy is too extensive. This finding was generally pervasive across all ypT stages and among groups with a variable actual number of lymph nodes resected.
Fewer lymph nodes are resected during esophagectomy in patients who have undergone induction therapy compared with those who undergo esophagectomy alone.3 Speculatively, this may be due to fibrosis of lymph nodes from induction therapy, specifically radiotherapy.3,23 These studies have been used to justify lymph node counts as low as a median of 8 lymph nodes resected. Our data suggest that when few lymph nodes are resected, there is a survival decrement, albeit not as great as lymphadenectomy at the opposite extreme.
Rizk and colleagues1 defined specific optimum lymph node counts per pT stage for esophagectomy alone in treatment-naïve patients. Our study shows that after induction therapy, such granularity by ypT stage is not needed. Specifically, that study recommended resecting 29–50 lymph nodes to maximize survival of patients with pT3/4 cancers. The present study shows that after induction therapy, there is a decrement, not a maximization, of survival with that great an extent of lymphadenectomy.
There is a stark contrast between the benefit of lymphadenectomy in treatment-naive (esophagectomy-only) patients and that in those who have undergone induction therapy. In patients experiencing a complete response (ypT0N0M0), extent of lymphadenectomy seems to have a limited correlation with survival. However, when residual disease is present (particularly ypN+), there is a benefit of more extensive lymphadenectomy up to a point. The decrement in survival with more extensive lymphadenectomy beyond about 30 nodes is a novel finding. The mechanism for the decrement is unclear. One can speculate that extensive lymphadenectomy occurs during radical resection, which might have increased morbidity; the WECC database lacks granularity to further examine this possibility. However, 30-day mortality was less than 2% in both N0 and N+ groups, and much of this mortality occurred in patients recorded as having no nodes resected. When zero lymph nodes were resected, one could speculate that the reasons may have been related to intraoperative complications or to findings that led to a palliative esophagectomy where oncological principles could not be followed.
Limitations
Data used in this analysis reflected real-world therapy for adenocarcinoma of the esophagus or esophagogastric junction from every inhabited continent4; however, such a multi-continent, multi-national, multi-institutional database is limited by lack of protocol standardization among institutions regarding extent of lymphadenectomy and pathologic review of the resection specimen. The method for counting lymph nodes resected may be institution specific, and some pathology laboratories may not have been as fastidious as others, thereby providing an artificially low count. The measure of optimum lymphadenectomy was risk-adjusted all-cause mortality. Despite the high lethality of esophageal cancer, all-cause mortality likely included a few non-cancer deaths. Nevertheless, it is a reliable endpoint, and when adjusted for comorbidities as in this study, may be more reliable than disease-specific survival24,25; it is also the basis for most cancer staging.26,27 We did not have morbidity information according to extent of lymphadenectomy, although 30-day mortality was low overall.4 Although the analyses performed in this study were within a machine-learning causal framework, the findings still represent correlations, not a causal relationship. We have no plausible biologic explanation for the nonlinear parabolic relationship identified.
Finally, a limitation of a study in which lymph node counts are recommended is that such information is available only after the fact. Intraoperatively, it is not possible to specifically count lymph nodes and stop at a certain point. The recommendations are meant to provide quality metrics for surgeons, pathologists, and programs to set benchmarks and to monitor frequency of outliers.
Recommendations
National Comprehensive Cancer Network guidelines suggest 15 lymph nodes as a number to optimize survival, and our data would suggest a higher number up to 25–30 lymph nodes (https://www.nccn.org/professionals/physician_gls/default.aspx#site). Knowing the value of the adequacy of lymph node counts allows one to set programmatic benchmarks for lymph node counts and increase the fastidiousness of both surgeon and pathologist. At Cleveland Clinic, we addressed the issue of adequacy of lymphadenectomy by deliberately sampling various lymph node stations, sending these as separate specimens for pathologic analysis. During discussions of the pathology of the cancer in tumor board meetings, number of lymph nodes resected is reported.
Conclusions
Lymphadenectomy should be performed in all esophageal resections after induction therapy, because there is a survival benefit to doing so. Based on analysis of worldwide data, for the purpose of maximizing survival we recommend the minimum bar for lymphadenectomy be raised from the commonly accepted 15 lymph nodes to a number closer to 25 to 30 nodes.
Supplementary Material
Acknowledgments
Sources of funding: Funded in part by the International Society for Diseases of the Esophagus; the Daniel and Karen Lee Chair in Thoracic Surgery at Cleveland Clinic; the Drs. Sidney and Becca Fleischer Heart and Vascular Education Chair; the Clinical and Translational Science Collaborative at the Case Western Reserve University School of Medicine, UL1TR000439, from the NIH National Center for Advancing Translational Sciences and NIH Roadmap for Medical Research; the National Institute of General Medical Sciences R01GM125072; and the Gus P. Karos Registry Fund at Cleveland Clinic. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the ISDE, Cleveland Clinic, or NIH.
Appendix 1.
Worldwide Esophageal Cancer Collaboration: Participating Institutions and Investigators
| Institution | Location | Investigators |
|---|---|---|
| Beijing Cancer Hospital, Peking University | Beijing, China | Ken N. Chen |
| Cleveland Clinic | Cleveland, OH; USA | Thomas W. Rice Eugene H. Blackstone |
| Case Western Reserve University | Cleveland, OH; USA | Carolyn Apperson-Hansen |
| Erasmus Medical Center | Rotterdam, The Netherlands | Bas P.L. Wijnhoven Jan van Lanschot Sjoerd Lagarde |
| Fourth Hospital of Hebei Medical University | Shijiazhuang, Hebei; China | Jun-Feng Liu |
| Fox Chase Cancer Center | Philadelphia, PA; USA | Walter J. Scott Donna Edmondson |
| Groote Schuur Hospital, University of Cape Town | Cape Town, South Africa | Riette Burger |
| Guy’s & St. Thomas’ Hospitals | London, UK | Andrew R. Davies Janine Zylstra |
| Helsinki University Hospital | Helsinki, Finland | Jari V. Räsänen Jarmo A. Salo Yvonne Sundstrom |
| Hospital Universitario del Mar | Barcelona, Spain | Manuel Pera |
| Hôpital Nord | Marseille, France | Xavier B. D’Journo |
| Indiana University Medical Center | Indianapolis, IN; USA | Kenneth A. Kesler |
| University of Texas MD Anderson Hospital | Houston, TX; USA | Wayne L. Hofstetter Arlene Correa Stephen G. Swisher |
| Mayo Clinic | Rochester, MN; USA | Mark S. Allen |
| Medical University of South Carolina | Charleston, SC; USA | Chad E. Denlinger |
| Memorial Sloan-Kettering Cancer Center | New York, NY; USA | Valerie W. Rusch |
| University of Queensland, Princess Alexandra Hospital | Brisbane, Australia | B. Mark Smithers David Gotley Andrew Barbour Iain Thomson |
| University of Newcastle upon Tyne | Newcastle upon Tyne, UK | S. Michael Griffin Jon Shenfine |
| Oregon Health & Science University | Portland, OR; USA | Paul H. Schipper John G. Hunter |
| Royal Marsden NHS Foundation Trust | London, UK | William H. Allum |
| Shanghai Chest Hospital | Shanghai, China | Wentao (Vincent) Fang |
| Toronto General Hospital | Toronto, ON; Canada | Gail E. Darling |
| University Zeikenhuizen Leuven | Leuven, Belgium | Tony E.M.R. Lerut Phillipe R. Nafteux |
| University Medical Center Utrecht | Utrech, The Netherlands | Richard van Hillegersberg |
| University of Alabama at Birmingham | Birmingham, AL; USA | Robert J. Cerfolio |
| Hospital de Clinicas, University of Buenos Aires | Buenos Aires, Argentina | Luis Durand Roberto De Antón |
| The University of Chicago, Department of Surgery | Chicago, IL; USA | Mark K. Ferguson |
| University of Hong Kong Medical Center, Queen Mary Hospital | Hong Kong, China | Simon Law |
| University of Michigan | Ann Arbor, MI; USA | Mark B. Orringer Becky L. Marshall |
| University of Montreal | Montreal, Quebec; Canada | André Duranceau Susan Howson |
| University of Pittsburgh Medical Center | Pittsburgh, PA; USA | James D. Luketich Arjun Pennathur Kathy Lovas |
| University of Rochester | Rochester, NY; USA | Thomas J. Watson |
| University of São Paulo | São Paulo, Brazil | Ivan Cecconello |
| West China Hospital of Sichuan University | Chengdu, Sichuan; China | Long-Qi Chen |
Footnotes
Conflicts of interest: None declared
REFERENCES
- 1.Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010;251:46–50. [DOI] [PubMed] [Google Scholar]
- 2.Samson P, Puri V, Broderick S, et al. Extent of lymphadenectomy is associated with improved overall survival after esophagectomy with or without induction therapy. Ann Thorac Surg. 2017;103:406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giugliano DN, Berger AC, Pucci MJ, et al. Comparative quantitative lymph node assessment in localized esophageal cancer patients after R0 resection with and without neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2017;21:1377–1384. [DOI] [PubMed] [Google Scholar]
- 4.Rice TW, Apperson-Hansen C, DiPaola LM, et al. Worldwide Esophageal Cancer Collaboration: clinical staging data. Dis Esophagus. 2016;29:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice TW, Chen LQ, Hofstetter WL, et al. Worldwide Esophageal Cancer Collaboration: pathologic staging data. Dis Esophagus. 2016;29:724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice TW, Ishwaran H, Blackstone EH, et al. Recommendations for clinical staging (cTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice TW, Ishwaran H, Hofstetter WL, et al. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rice TW, Ishwaran H, Kelsen DP, et al. Recommendations for neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice TW, Lerut TE, Orringer MB, et al. Worldwide Esophageal Cancer Collaboration: neoadjuvant pathologic staging data. Dis Esophagus. 2016;29:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman AI. Eventcharts: visualizing survival and other timed-event data. Am Statistician. 1992;46:13–18. [Google Scholar]
- 11.Meinshausen N Quantile regression forests. J Machine Learning Res. 2006;7:983–999. [Google Scholar]
- 12.Breiman L Random forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 13.Ishwaran H, Kogalur UB. RandomForestSRC: Random forests for survival, regression and classification (RF-SRC). R package version 2.6.0.13. http://cran.r-project.org, 2018. [Google Scholar]
- 14.Lu M, Sadiq S, Feaster DJ, et al. Estimating individual treatment effect in observational data using random forest methods. J Comput Graph Stat. 2018;27:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royston P, Parmar MK. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med. 2011;30:2409–2421. [DOI] [PubMed] [Google Scholar]
- 16.Andersen PK, Hansen MG, Klein JP. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 2004;10:335–350. [DOI] [PubMed] [Google Scholar]
- 17.Irwin JO. The standard error of an estimate of expectation of life, with special reference to expectation of tumourless life in experiments with mice. J Hyg (Lond). 1949;47:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang F, Ishwaran H. Random forest missing data algorithms. Stat Analysis Data Mining. 2017;10:363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koen Talsma A, Shapiro J, Looman CW, et al. Lymph node retrieval during esophagectomy with and without neoadjuvant chemoradiotherapy: prognostic and therapeutic impact on survival. Ann Surg. 2014;260:786–792; discussion 792–783. [DOI] [PubMed] [Google Scholar]
- 20.Ishwaran H, Blackstone EH, Apperson-Hansen C, et al. A novel approach to cancer staging: application to esophageal cancer. Biostatistics 2009;10:603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rusch VW, Rice TW, Crowley J, et al. The seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals: the new era of data-driven revisions. J Thorac Cardiovasc Surg.139:819–821. [DOI] [PubMed] [Google Scholar]
- 22.Rice TW, Gress DM, Patil DT, et al. Cancer of the esophagus and esophagogastric junction: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:304–317. [DOI] [PubMed] [Google Scholar]
- 23.Hanna JM, Erhunmwunsee L, Berry M, et al. The prognostic importance of the number of dissected lymph nodes after induction chemoradiotherapy for esophageal cancer. Ann Thorac Surg. 2015;99:265–269. [DOI] [PubMed] [Google Scholar]
- 24.van Leeuwen PJ, Kranse R, Hakulinen T, et al. Disease-specific mortality may underestimate the total effect of prostate cancer screening. J Med Screen. 2010;17:204–210. [DOI] [PubMed] [Google Scholar]
- 25.Black WC, Haggstrom DA, Welch HG. All-cause mortality in randomized trials of cancer screening. J Natl Cancer Inst. 2002;94:167–173. [DOI] [PubMed] [Google Scholar]
- 26.Goense L, Visser E, Haj Mohammad N, et al. Role of neoadjuvant chemoradiotherapy in clinical T2N0M0 esophageal cancer: a population-based cohort study. Eur J Surg Oncol. 2018;44:620–625. [DOI] [PubMed] [Google Scholar]
- 27.Markar SR, Gronnier C, Pasquer A, et al. Role of neoadjuvant treatment in clinical T2N0M0 oesophageal cancer: results from a retrospective multi-center European study. Eur J Cancer. 2016;56:59–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.