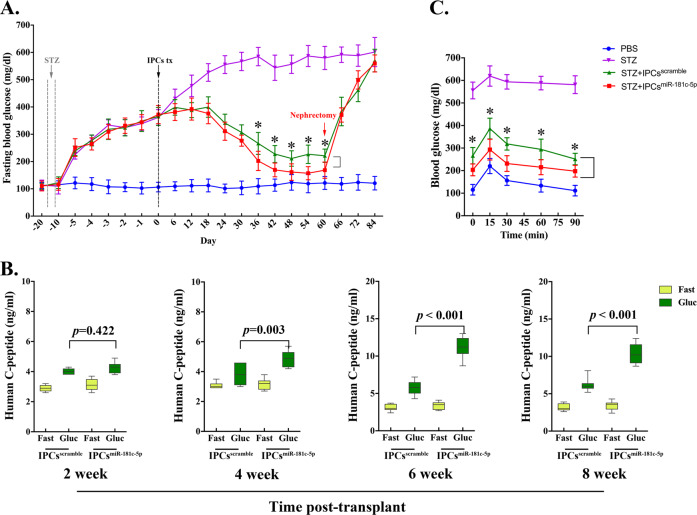
Fig. 3. Transplanted insulin-producing cells ameliorate hyperglycemia and remain glucose responsive in diabetic mice.
Mice were treated with the mouse-specific beta-cell toxin streptozotocin (STZ, 35 mg/kg via intraperitoneal injection for 5 days) to ablate endogenous beta cells. Insulin-producing cells (~1 × 107 cells/animal) were transplanted under the kidney capsule of diabetic mice. a Fast blood glucose was assessed every 6 days throughout the study and a survival nephrectomy was performed on all mice on day 60 to remove the engrafted kidney (indicated by red arrow). Unilateral nephrectomy of IPCs graft-bearing mice resulted in a rapid rise in fast blood glucose levels, directly demonstrating euglycemic control due to IPCs grafts after STZ treatment in these mice (n = 7). IPCsscramble, control hiPSCs-derived insulin-prodcuing cells. IPCsmiR-181c-5p, miR-181c-5p overexpressed hiPSCs-derived insulin-prodcuing cells. *p ≤ 0.05, STZ + IPCsmiR-181c-5p group compares with STZ + IPCsscramble group. b ELISA analysis of serum from fasted and glucose-challenged (3 g/kg) mice post transplantation. IPCs graft-bearing mice exhibit significant higher levels of circulating human C-peptide in serum after glucose challenge, indicating that transplanted IPCs cells remain functional in vivo. Mice transplanted with miR-181c-5p overexpressed hiPSCs-derived IPCs exhibit significant higher levels of human C-peptide at 4-week, 6-week, and 8-week post transplantation. c Blood glucose levels were measured during an intraperitoneal injection glucose tolerance test at 8-week post transplantation. *p ≤ 0.05, STZ + IPCsmiR-181c-5p group compares with STZ + IPCsscramble group.