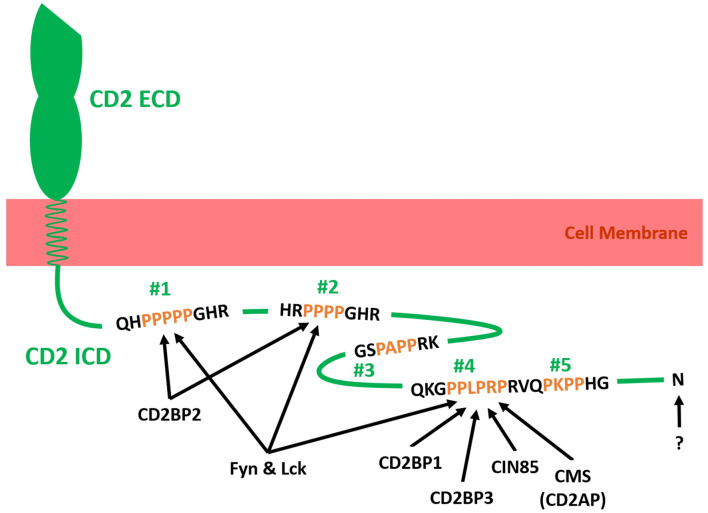
Figure 2.
Schematic illustration of adaptor molecules binding to the intracellular tail domain (ICD) of human CD2 (Green). The ICD of CD2 is connected to the extracellular domain (ECD) of CD2 via a transmembrane helix in the cell membrane (red). The ICD of CD2 contains five SH3 binding domains (Two proline residues separated by two or three amino acids and flanked by a basic amino acid residue; #1–#5), of which two can also act as GYF-binding motifs (#1 and #2). Fyn kinase and CD2-binding protein 2 (CD2BP2) have been shown to bind to #1 and #2 (41). Additionally, Fyn kinase binds to #4 and/or #5 (42). Lck kinase has been shown to interact with #1, #2 and #4 in rat CD2 ICD (43). CD2BP1, CD2BP3, Cbl-interacting protein of 85 kDa (CIN85) and Cas ligand with multiple SH3 domains (CMS; Human form of CD2-associated protein CD2AP) bind to #4 (44, 45). The C-terminal amino acid N327 has been shown to be essential for CD2-LFA3 affinity regulation but no binding partner has been identified (38, 46). The ICD of CD2 precipitated with the T cell receptor complex (TCR/CD3) and Phosphatidylinositol-3 kinase (PI3K) but no direct interaction and binding motif has been identified (47, 48). Further direct interactions between the cytoplasmic tail of CD2 and intracellular or membrane proteins may exist but have yet to be identified.