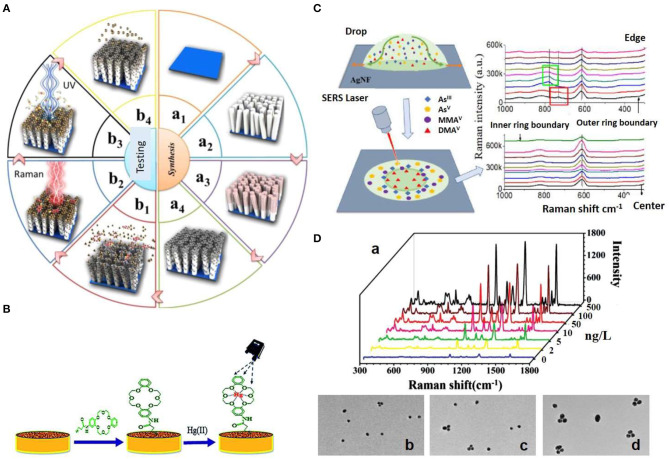
Figure 2.
Strategies for SERS detection of inorganic pollutants. (A) Schematic illustration of the fabrication of ZnO/Ag nanoarrays for the Hg2+ ions SERS detection. (a) The ZnO/Ag nanoarrays were fabricated by growing ZnO nanoarrays via soft hydrothermal method (a1, a2) and the subsequent deposition of Ag nanoparticles via an electroless plating technique (a3, a4). (b) Adsorption of Hg2+ ions and subsequent adsorption of Rhodamine B (RB) on the on ZnO/Ag nanoarrays (b1), Hg2+ ions detection via SERS monitoring of RB (b2), photocatalytic degradation of RB (b3), and Hg2+ removal via heat treatment (b4). Reproduced from Esmaielzadeh Kandjani et al. (2015) with permission from American Chemical Society (Copyright 2015). (B) Schematic representation of the modification of nanostructured Au substrate with a crown ether derivative for capturing Hg2+. Reproduced from Sarfo et al. (2017) with permission from The Royal Society of Chemistry. (C) SERS method for arsenic speciation by using the separation potential of the coffee ring effect on negatively charged Ag nanofilms. Reproduced from Yang et al. (2019) with permission from American Chemical Society (Copyright 2019). (D) Triple Raman label-encoded Au NPs trimer for heavy metal ion detection. (a) SERS spectra under the same concentration of Hg2+ and Ag+ ranging from 0 to 500 ng/L. (b–d) TEM images of Au NP trimers assembled by the addition of equal concentration of Hg2+ and Ag+ at different concentrations: 5 (b), 50 (c), and 100 (d) ng/L. Reproduced from Li et al. (2015) with permission from John Wiley & Sons (Copyright 2015).