Abstract
Background
Obesity, a known risk factor for chronic kidney disease (CKD), is generally assessed using body mass index (BMI). However, BMI may not effectively reflect body composition, and the impact of muscle‐to‐fat (MF) mass balance on kidney function has not been elucidated. This study evaluated the association between body muscle and fat mass balance, represented as the MF ratio, and incident CKD development.
Methods
Data were retrieved from a prospective community‐based cohort study (Korean Genome and Epidemiology Study). Muscle and fat mass were measured using multifrequency bioelectrical impedance analysis. The study endpoint was incident CKD (estimated glomerular filtration rate <60 mL/min/1.73 m2 in at least two or more consecutive measurements during the follow‐up period).
Results
Totally, 7682 participants were evaluated. Their mean age was 51.7 ± 8.7 years, and 48% of the subjects were men. During a median follow‐up of 140.0 (70.0–143.0) months, 633 (8.2%) subjects developed incident CKD. When the association between body composition and incident CKD was investigated, multivariable Cox proportional hazard analysis revealed that increase in MF ratio was related with a decreased risk of CKD development [per 1 increase in MF ratio: hazard ratio (HR), 0.86; 95% confidence interval (CI), 0.77–0.96; P = 0.008]. This association was also maintained when MF ratio was dichotomized according to sex‐specific median values (high vs. low: HR, 0.83; 95% CI, 0.70–0.98; P = 0.031). Analyses preformed in a propensity score matched group also revealed a similar decreased risk of incident CKD in high MF ratio participants (high vs. low: HR, 0.84; 95% CI, 0.71–0.98; P = 0.037). This relationship between MF ratio and incident CKD risk was consistently significant across subgroups stratified by age, sex, hypertension, estimated glomerular filtration rate categories, and proteinuria. Among different BMI groups (normal, overweight, and obese), the relationship between high MF ratio and lower incident CKD risk was significant only in overweight and obese subjects.
Conclusions
Lower fat mass relative to muscle mass may lower the risk of CKD development in individuals with normal renal function. This relationship seems more prominent in overweight and obese subjects than in normal weight subjects.
Keywords: Muscle‐to‐fat ratio, Body mass index, Muscle mass, Fat mass, Obesity, Chronic kidney disease
Introduction
Obesity is considered a major public health problem owing to its rapidly increasing prevalence and close association with poor outcomes.1 A commonly used measure for assessing adiposity is body mass index (BMI).2 Studies have shown BMI to be a significant risk factor for the development of various acute and chronic conditions such as hypertension, type 2 diabetes, cardiovascular diseases (CVDs), and certain types of cancer.3 Several recent large epidemiologic investigations have also reported that the risk of chronic kidney disease (CKD) development clearly increases with the increase in BMI.4 However, others have failed to observe such associations.5, 6
One major shortcoming of using BMI to determine obesity is that BMI does not account for body composition.7, 8 BMI, calculated based only on height and weight, is an inaccurate measure of body fat content and does not take into account the fluid status, muscle mass, or bone density.9, 10 Individuals with the same BMI may largely vary in body composition. This inaccuracy of BMI in representing adiposity may be one of the reasons for the controversial results among studies evaluating the association between BMI and CKD development.
Recent studies have suggested that body fat mass and muscle mass may differently affect health outcomes. An increase in muscle mass has been linked to less insulin resistance and protection against the development of type 2 diabetes.11, 12, 13 In addition, a lower amount of lean body mass has been reported to be related to an increased risk of cardiovascular events.14, 15 On the other hand, increased fat mass has been shown to increase the risk of metabolic derangements and poor cardiovascular outcome.16, 17 Moreover, the substantial loss of muscle mass relative to fat mass, termed as ‘sarcopenia’, has been found to have a negative effect on various cardiometabolic parameters and on mortality.18, 19, 20 This relationship has been observed not only in the elderly population and in patients with cancer, in whom muscle loss is prevalent, but also in the general population.10, 21 Nonetheless, the impact of muscle mass to fat mass balance on kidney function has not been clearly elucidated.
Therefore, the present study aimed to evaluate the association between the balance of body muscle and fat mass composition, represented as the muscle‐to‐fat (MF) ratio, and incident CKD development in a prospective community‐based cohort of subjects with normal renal function.
Materials and methods
Study subjects
This study used data from the Korean Genome and Epidemiology Study (KoGES), a prospective community‐based cohort study. The detailed profile and methods of how the KoGES cohort was assembled were previously described elsewhere.22 Briefly, the study cohort consisted of 10 030 subjects aged 40–69 years who were residents of Ansan or Ansung, which are cities near the capital city of Seoul, South Korea. The subjects underwent medical health evaluations and various surveys at baseline. Serial health examinations and surveys were performed biennially from 2001 to 2014. In this study, subjects with available body composition data were initially screened for inclusion. Subsequent exclusions were performed for those with an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 or underlying kidney disease at baseline, those with missing data, and those with missing follow‐up creatinine data. A total of 7682 subjects were included in the final analysis (Supporting Information, Figure S1). All subjects voluntarily participated in the study and provided informed consent. The study protocol was approved by the Ethics Committee of KoGES at the Korean National Institute of Health. This study was performed in accordance with the Declaration of Helsinki and approved by the institutional review board of Yonsei University Health System Clinical Trial Center (4‐2016‐0900).
Anthropometric and laboratory data
All subjects underwent a comprehensive medical health examination and completed questionnaires about health and lifestyle factors at the time of study entry. Demographic and socio‐economic data, including age, sex, education and income level, smoking status, alcohol intake, and medical histories, were obtained. Anthropometric parameters such as height and weight were measured by trained researchers according to a specific protocol in which the subjects took off their shoes, wore light clothing, and stood on a scale on an even surface while measurements were taken. Education level was classified into three groups: low, lower than middle school; middle, middle school; and high, higher than middle school. Income level was divided into tertile groups based on the average per‐person monthly income: low, <$850 per month; middle, $850–1700 per month; and high, ≥$1700 per month. Physical activity status was categorized into active (at least 30 min/day of moderate‐intensity activity) or inactive. Subjects who had a blood pressure of ≥140/90 mmHg or were taking antihypertensive agents were considered to have hypertension. Those who had a blood glucose level of ≥126 mg/dL in an 8 h fasting status, had a post‐load glucose level of ≥200 mg/dL after a 75 g oral glucose tolerance test, had a haemoglobin A1c (HbA1c) value of ≥6.5%, or were taking oral medication and/or receiving insulin treatment for hyperglycaemia were considered to have diabetes. Subjects who had a medical history of dyslipidaemia or were taking medications for lipid control were considered to have dyslipidaemia. CVDs were defined as the composite of myocardial infarction, congestive heart failure, and coronary artery disease.
Blood and urine samples were obtained after an 8 h fast and transported to a central laboratory (Seoul Clinical Laboratories, Seoul, Republic of Korea) within 24 h of sampling. The serum concentrations of blood urea nitrogen, creatinine, albumin, glucose, total cholesterol, triglyceride, high‐density lipoprotein cholesterol (HDL‐C), and C‐reactive protein (CRP) were measured using ADVIA 1650 (Siemens, Tarrytown, NY, USA). Serum creatinine level was measured using the Jaffé method throughout the study period. The creatinine levels were reduced by a calibration factor of 5% for standardization to isotope dilution mass spectrometry reference method values.23 Low‐density lipoprotein cholesterol (LDL‐C) level was calculated using the following formula: [total cholesterol (mg/dL) − HDL‐C (mg/dL) − triglyceride (mg/dL) /5]. The HbA1c level was determined using high‐performance liquid chromatography (Variant II; BioRad Laboratories, Hercules, CA, USA). Haemoglobin levels were measured using an autoanalyser (Sysmex, Kobe, Japan). Homeostatic model assessment of insulin resistance (HOMA‐IR) was calculated by following formula: [fasting insulin (uU/mL) × fasting plasma glucose (mg/dL) /405]. Urine samples were collected in the morning after the first voiding and subjected to a dipstick test using URISCAN Pro II (YD Diagnostics Corp., Seoul, Korea). Proteinuria was quantified as absent, trace, 1+, 2+, or 3+ based on a colour scale. The presence of proteinuria was defined as a dipstick urine test result of more than or equal to the trace level.
Assessment of body composition
Body composition was assessed using multifrequency bioelectrical impedance analysis (BIA; InBody 3.0, Biospace, Seoul, Korea). Compared with conventional BIA‐based analysis methods that rely on formulae to calculate the estimated mass of each body component, multifrequency BIA assumes that the human body consists of five interconnecting cylinders and performs impedance measurements directly on these compartments. Using a tetrapolar 8‐point tactile electrode system, impedances were measured at four specific frequencies (5, 50, 250, and 500 kHz) in five segments (right arm, left arm, trunk, right leg, and left leg). Muscle mass and fat mass were expressed as the muscle mass index (MMI, muscle mass/height2) and the fat mass index (FMI, fat mass/height2), respectively. The MF ratio was defined as MMI/FMI. BMI was calculated as weight divided by height squared (kg/m2). Subjects were allocated into two groups based on the sex‐specific median values of the MF ratio. Sex‐specific median value of the MF ratio was defined as the median of the MF ratio in men and women, separately. The World Health Organization obesity classification for Asian populations was used to determine overweight and obesity.
Study outcome
The primary endpoint was incident CKD development. CKD was defined as an eGFR of <60 mL/min/1.73 m2 in at least two or more consecutive measurements during the follow‐up period. The eGFR was calculated using the CKD Epidemiology Collaboration equation.24
Statistical analysis
All statistical analyses were performed using IBM SPSS software for Windows version 23.0 (IBM Corporation, Armonk, NY, USA), SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA), and R software 3.3.1 (http://www.R‐project.org). Continuous variables were expressed as means ± standard deviations, and categorical variables were expressed as absolute numbers with percentages. All data were tested for normality before statistical analysis. The Kolmogorov–Smirnov test was performed to determine the normality of the distribution of the parameters. Intergroup comparisons were performed using analysis of variance or Student's t‐test for normally distributed continuous variables, whereas categorical variables were examined using the χ 2 test or Fisher's exact test. Data that did not show normal distribution were presented as medians with interquartile ranges and compared using the Mann–Whitney U test or Kruskal–Wallis test. Cumulative renal survival rates were estimated using Kaplan–Meier analyses and log‐rank tests. Survival time was defined as the time interval between baseline and the onset of outcome or the last follow‐up. Subjects who were lost during follow‐up were censored in the final analysis. Cox proportional hazard models were constructed to determine the independent predictive value of the MF ratio for incident CKD development. Variables that presented statistical significance in the univariable analysis were included in the multivariable models (Supporting Information, Table S1 ). Model 1 was not adjusted for any covariates. Model 2 included baseline age and sex. Model 3 included demographic factors and co‐morbidities. Model 4 was further adjusted for laboratory parameters. For the propensity score matching (PSM) analyses, the propensity score was determined using binary logistic regression with greedy nearest neighbour matching technique without replacement. A caliper of 0.2 times the standard deviation was used. Subjects with high MF ratio were matched to those with low MF ratio. In the matched cohort, the groups were compared with paired t‐test and McNemar test, as appropriate. To test the relationship between incident CKD risk and MF ratio as a continuous variable, restricted cubic spline analyses were conducted. Extreme outliers, defined as values less than first quartile (Q1) − 1.5 × interquartile range (Q3–Q1) or greater than third quartile (Q3) + 1.5 × interquartile range, were excluded when depicting the cubic spline analysis results. For sensitivity analysis, analyses were conducted with creatinine levels adjusted through a conversion equation different from the formula used in the main analyses.25, 26 P‐values of <0.05 were considered statistically significant.
Results
Baseline characteristics
The baseline characteristics of the study subjects according to the sex‐specific median values of the MF ratio are described in Table 1. The median MF ratio was 3.4 [2.8–4.2] and 2.0 [1.7–2.3] in male and female participants, respectively. The mean age was 51.4 ± 8.7 years, and 3686 (48%) subjects were men. The mean eGFR was 93.9 ± 14.2 mL/min/1.73 m2. The MMI, FMI, BMI, and waist‐to‐hip ratio were significantly lower in the high MF ratio group than in the low MF ratio group. Subjects in the high MF ratio group were younger and were more likely to be physically active than those in the low MF ratio group. However, the proportions of men, smokers, and alcohol drinkers were comparable between the groups. Subjects in the high MF ratio group were more likely to engage in physical activities than those in the low MF ratio group. The systolic blood pressure (SBP) was lower, and fewer subjects had co‐morbidities such as hypertension, diabetes, dyslipidaemia, and CVDs in the high MF ratio group than in the low MF ratio group. With respect to laboratory test results, eGFR was higher and the proportion of proteinuria‐positive subjects was lower in the high MF ratio group than in the low MF ratio group. The levels of haemoglobin, albumin, total cholesterol, LDL‐C, triglyceride, fasting plasma glucose, HbA1c, HOMA‐IR, and CRP were significantly lower in the high MF ratio group than in the low MF ratio group.
Table 1.
Baseline characteristics according to sex‐specific median of the muscle‐to‐fat ratio
| Characteristics | Sex‐specific MF ratio | |||
|---|---|---|---|---|
|
Total (n = 7682) |
Low (n = 3839) |
High (n = 3843) |
P | |
| Body composition | ||||
| MF ratio | 2.9 ± 1.2 | 2.2 ± 0.6 | 3.5 ± 1.3 | <0.001 |
| Muscle mass index (kg/m2) | 16.8 ± 1.7 | 17.2 ± 1.7 | 16.4 ± 1.7 | <0.001 |
| Fat mass index (kg/m2) | 6.8 ± 2.4 | 8.3 ± 2.1 | 5.2 ± 1.6 | <0.001 |
| BMI (kg/m2) | 24.6 ± 3.1 | 26.5 ± 2.6 | 22.7 ± 2.2 | <0.001 |
| WHR | 0.87 ± 0.08 | 0.90 ± 0.07 | 0.85 ± 0.07 | <0.001 |
| Demographic data | ||||
| Age (years) | 51.4 ± 8.7 | 52.3 ± 8.7 | 50.4 ± 8.6 | <0.001 |
| Male, n (%) | 3686 (48.0) | 1846 (48.1) | 1840 (47.9) | 0.437 |
| Smoking status, n (%) | 3129 (41.2) | 1533 (40.4) | 1596 (42.0) | 0.083 |
| Alcohol status, n (%) | 4143 (54.4) | 2050 (53.8) | 2093 (54.9) | 0.173 |
| Physical activity status, n (%) | 3796 (50.7) | 1821 (48.6) | 1975 (52.8) | <0.001 |
| Physical activity (Mets) | 9095.1 ± 6037.2 | 8492.5 ± 5736.9 | 9697.1 ± 6266.1 | <0.001 |
| SBP (mmHg) | 121.0 ± 18.4 | 124.3 ± 18.7 | 117.6 ± 17.5 | <0.001 |
| Education, n (%) | <0.001 | |||
| Low | 2282 (29.9) | 1223 (32.1) | 1059 (27.7) | |
| Intermediate | 4214 (55.3) | 1999 (52.5) | 2215 (58.0) | |
| High | 1130 (14.8) | 584 (15.3) | 546 (14.3) | |
| Income, n (%) | 0.372 | |||
| Low | 2357 (31.2) | 1196 (31.7) | 1161 (30.6) | |
| Intermediate | 2185 (28.9) | 1064 (28.2) | 1121 (29.6) | |
| High | 3019 (39.9) | 1512 (40.1) | 1507 (39.8) | |
| Co‐morbidities, n (%) | ||||
| Hypertension | 1071 (13.9) | 740 (19.3) | 331 (8.6) | <0.001 |
| Diabetes | 493 (6.4) | 294 (7.7) | 199 (5.2) | <0.001 |
| Dyslipidaemia | 203 (2.6) | 122 (3.2) | 81 (2.1) | 0.002 |
| CVDs | 105 (1.4) | 70 (1.8) | 35 (0.9) | <0.001 |
| Laboratory data | ||||
| eGFR (mL/min/1.73 m2) | 93.9 ± 14.2 | 92.9 ± 14.1 | 94.9 ± 14.3 | <0.001 |
| Proteinuria (%) | 581 (7.6) | 321 (8.4) | 260 (6.8) | 0.005 |
| Haemoglobin (g/dL) | 13.6 ± 1.6 | 13.8 ± 1.5 | 13.4 ± 1.6 | <0.001 |
| Albumin (g/dL) | 4.52 ± 0.28 | 4.53 ± 0.28 | 4.51 ± 0.29 | 0.003 |
| Total cholesterol (mg/dL) | 199.1 ± 36.7 | 206.3 ± 36.9 | 191.8 ± 35.1 | <0.001 |
| LDL‐C (mg/dL) | 119.2 ± 34.5 | 124.1 ± 35.0 | 114.2 ± 33.2 | <0.001 |
| HDL‐C (mg/dL) | 49.5 ± 11.8 | 47.5 ± 10.7 | 51.6 ± 12.4 | <0.001 |
| Triglyceride (mg/dL) | 151.8 ± 108.3 | 173.5 ± 119.0 | 130.1 ± 91.3 | <0.001 |
| Fasting glucose (mg/dL) | 92.6 ± 23.2 | 95.2 ± 24.7 | 90.0 ± 21.2 | <0.001 |
| HbA1c (%) | 5.8 ± 0.9 | 5.9 ± 0.9 | 5.7 ± 0.8 | <0.001 |
| HOMA‐IR | 1.7 ± 1.2 | 2.0 ± 1.4 | 1.5 ± 1.1 | <0.001 |
| CRP [IQR] (mg/dL) | 0.14 [0.06–0.25] | 0.17 [0.09–0.28] | 0.12 [0.05–0.20] | <0.001 |
Data are presented as mean (standard deviation), median [interquartile range], or number (%). Sex‐specific median of the MF ratio was 3.4 [2.8–4.2] in men and 2.0 [1.7–2.3] in women. BMI, body mass index; CRP, C‐reactive protein; CVDs, cardiovascular diseases; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; HbA1c, haemoglobin A1c; HOMA‐IR, homeostatic model assessment of insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; MF ratio, muscle‐to‐fat ratio; SBP, systolic blood pressure; WHR, waist‐to‐hip ratio.
When the baseline characteristics were compared between male and female participants, male subjects were younger. In addition, male participants had a higher MF‐ratio, MMI, and WHR. The FMI and BMI were lower in males compared to female participants. Male participants were more likely to smoke and drink alcohol and have a higher education and income than female participants. In addition, hypertension was more common while diabetes and dyslipidaemia were less common among men than women. Regarding laboratory test results, eGFR, LDL‐C, and HDL‐C levels were lower while haemoglobin, serum albumin, triglyceride, fasting glucose, HOMA‐IR, and CRP levels were higher among men than among women (Supporting Information, Table S2).
Relationship between muscle‐to‐fat ratio and metabolic factors
The associations between body composition indices and metabolic variables are shown in Supporting Information, Table S3. BMI revealed a positive relationship with SBP and total cholesterol, LDL‐C, triglyceride, HOMA‐IR, and CRP levels, whereas age and eGFR were negatively associated with each other. The MF ratio showed a positive association with serum albumin level, whereas a negative association was found among age, SBP, eGFR, and total cholesterol, LDL‐C, triglyceride, HOMA‐IR, and CRP levels.
When the relationship between body composition components and BMI was evaluated, a strong negative correlation was found between the MF ratio and BMI (β = −0.560, Supporting Information, Figure S2A). However, both the MMI and FMI showed a positive association with BMI (β = 0.618 and β = 0.809, respectively; Supporting Information, Figure S2B and S2C).
Association between muscle‐to‐fat ratio and chronic kidney disease development
During a median follow‐up of 140.0 (70.0–143.0) months, 633 (8.2%) subjects developed incident CKD. The follow‐up duration was similar between the high and low MF ratio groups [140.0 (69.9–143.0) vs. 141.0 (71.0–143.1) months, P = 0.140]. Cox proportional hazard analyses were performed to assess the association between the MF ratio and CKD development. When BMI was dichotomized according to the sex‐specific median values, CKD development risk was significantly increased in the high BMI group compared with the low BMI group in the unadjusted model [hazard ratio (HR), 1.37; 95% confidence interval (CI), 1.17–1.60]. However, when adjustments were made for confounding factors, the elevated CKD risk in the high BMI group was no longer significant (HR, 1.10; 95% CI, 0.93–1.31). On the other hand, CKD development risk was significantly decreased in the high MF ratio group than in the low MF ratio group, even after adjusting for confounding variables (HR, 0.83; 95% CI, 0.70–0.98). A similar association was found when MF ratio was considered as a continuous variable (Table 2). When restricted cubic spline analyses were further conducted, CKD development risk was noted to gradually decrease significantly with the increase of MF ratio (Supporting Information, Figure S3).
Table 2.
Risk of chronic kidney disease development according to body composition indices
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| BMI | ||||||||
| Per 1 kg/m2 increase | 1.08 (1.05–1.11) | <0.001 | 1.08 (1.06–1.11) | <0.001 | 1.05 (1.02–1.08) | <0.001 | 1.04 (0.99–1.08) | 0.059 |
| High vs. low | 1.37 (1.17–1.60) | <0.001 | 1.48 (1.26–1.73) | <0.001 | 1.22 (1.04–1.45) | 0.017 | 1.10 (0.93–1.31) | 0.245 |
| MF ratio | ||||||||
| Per 1 increase | 0.75 (0.69–0.82) | <0.001 | 0.74 (0.67–0.82) | <0.001 | 0.82 (0.74–0.91) | <0.001 | 0.86 (0.77–0.96) | 0.008 |
| High vs. low | 0.68 (0.58–0.80) | <0.001 | 0.68 (0.58–0.79) | <0.001 | 0.80 (0.67–0.94) | 0.007 | 0.83 (0.70–0.98) | 0.031 |
Model 1: unadjusted model; Model 2: adjusted for age and sex; Model 3: adjusted for Model 2 + systolic blood pressure, smoking status, alcohol intake, education levels, income levels, history of hypertension or diabetes, and physical activity; and Model 4: adjusted for Model 3 + estimated glomerular filtration rate, proteinuria, total cholesterol, and C‐reactive protein. BMI, body mass index; CI, confidence interval; HR, hazard ratio; MF ratio, muscle‐to‐fat ratio.
In order to further minimize the effect of confounding factors, the risk for incident CKD was further determined in 3280 participants matched by propensity score. As shown in Supporting Information, Table S4, the high and low MF ratio groups were well matched for baseline characteristics after PSM. Cox proportional hazard analyses preformed in the PSM group revealed that participants with a high MF ratio were associated with a significantly decreased risk of incident CKD compared with those with a low MF ratio (HR, 0.84; 95% CI, 0.71–0.98), a finding consistent with the result of the non‐matched cohort (Supporting Information, Table S5).
For sensitivity analysis, evaluations were also performed with creatinine levels that were adjusted using a conversion equation different from the formula used in the main analyses. The sensitivity analysis results were consistent with the main findings of the study (Supporting Information, Table S6).
Relationship of muscle‐to‐fat ratio with chronic kidney disease incidence in different body mass index groups
Further evaluations were made to assess the impact of the MF ratio in overweight and obese subgroups classified based on the BMI criteria. When the subjects were classified into normal weight (BMI <23.0 kg/m2), overweight (BMI 23.0–27.4 kg/m2), and obese (BMI ≥27.5 kg/m2) groups, a significant gradual increase in the risk of incident CKD development was found in those with a low MF ratio. However, in those with a high MF ratio, the risk of CKD development in overweight and obese subjects was comparable with that in subjects with normal BMI (Figure 1). In addition, the time to CKD development was significantly longer in those with a high MF ratio. This advantage against incident CKD development was maintained in overweight and obese subjects, but not in those with a normal BMI (Supporting Information, Figure S4). When the incidences of CKD were stratified against the sex‐specific median values of the MF ratio and the BMI group, the CKD incidence rate was the highest in obese subjects with a low MF ratio and the lowest in those with normal BMI and a high MF ratio (Figure 2).
Figure 1.
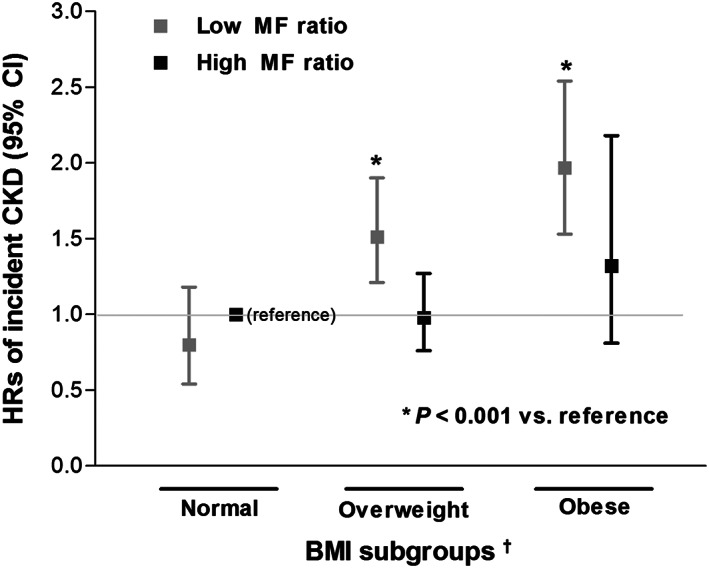
Comparison of risk for CKD development according to combination of BMI and sex‐specific median values of the MF ratio (low BMI with high MF ratio as reference group). BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; HRs, hazard ratios; MF ratio, muscle‐to‐fat ratio. †World Health Organization obesity classification for Asian population was used: normal (BMI <23.0 kg/m2), overweight (BMI 23.0–27.4 kg/m2), and obese (BMI ≥27.5 kg/m2).
Figure 2.
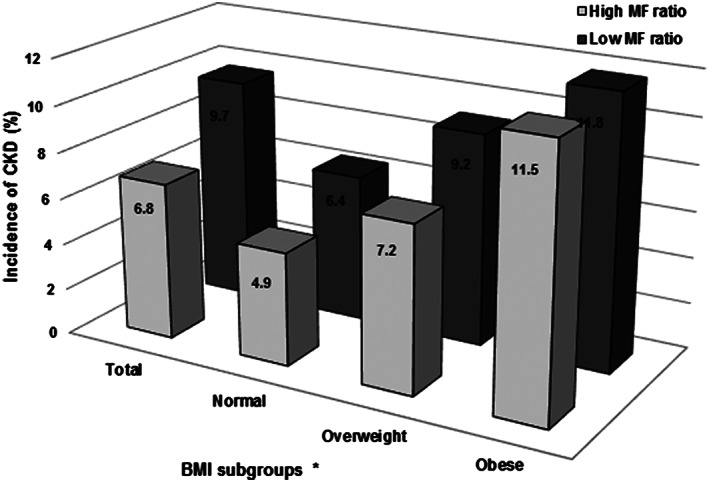
The prevalence of CKD according to sex‐specific median of the MF ratio in different BMI groups (P for trend <0.001). BMI, body mass index; CKD, chronic kidney disease; MF ratio, muscle‐to‐fat ratio. *World Health Organization obesity classification for Asian population was used: normal (BMI <23.0 kg/m2), overweight (BMI 23.0–27.4 kg/m2), and obese (BMI ≥27.5 kg/m2).
Subgroup analysis
The relationship between the MF ratio and incident CKD was further evaluated in subgroups stratified by age (<50 vs. ≥50 years), sex (female vs. male), hypertension (yes vs. no), eGFR (60–79.9 vs. 80–99.9 vs. ≥100 mL/min/1.73 m2), and proteinuria (yes vs. no). No significant interactions were found in any of the subgroups, suggesting that the relation between the MF ratio and incident CKD risk was consistently significant across these subgroups (Supporting Information, Figure S5).
Follow‐up loss
The overall follow‐up loss rate during the whole study duration was 29.5%. However, more than 62% of the subjects remained in the study for over 125 months (Supporting Information, Figure S6). The proportion of subjects lost to follow‐up was comparable between the high and low MF ratio groups [1170 (30.5%) vs. 1093 (28.4%), P = 0.051]. Changes in address or telephone number, being too busy to attend, and not answering telephone calls were the main reasons of follow‐up loss. Cumulative death rate between the groups was also comparable [367 (9.6%) vs. 336 (8.7%), P = 0.114].
Discussion
In this study, the association of BMI and the MF ratio with CKD development was investigated in a general population cohort with preserved kidney function. Metabolic indices were positively correlated with BMI and negatively correlated to the MF ratio. BMI did not show a significant relationship with CKD development after full adjustment, whereas the increase in the MF ratio was clearly associated with a decreased risk of CKD development. In addition, the impact of the MF ratio on CKD development was more definite in overweight or obese subjects than in those with a normal BMI.
Previous studies evaluating the effect of adiposity on CKD development have shown conflicting results. Obesity defined by BMI was significantly associated with an increased risk of incident CKD in most of the studies. This association was observed not only in cases when increased BMI was accompanied by deranged metabolic features such as dyslipidaemia and diabetes but also in metabolically healthy individuals who do not have features of metabolic disease other than being overweight.27, 28, 29 However, several other studies failed to show such a relationship. In a prospective cohort study (the Framingham Offspring Study), increased BMI itself was not an independent risk factor for stage 3 CKD development.6 In addition, an observational study of Japanese subjects without kidney disease showed that obese individuals without metabolic complications were not exposed to a higher risk of incident CKD compared with non‐obese healthy subjects.30 These controversies could be based on the fact that BMI is a surrogate measure with considerable limitations in assessing adiposity.7, 8 As BMI is not a surrogate of fat mass alone but represents the composite of fat, muscle, bone, and fluid, assessing the effect of each component of BMI is not possible.31 This limitation of BMI should be further emphasized on the basis of recent reports showing that excessive adiposity is metabolically harmful whereas muscle mass plays a beneficial role. The findings of this study demonstrating that BMI correlates positively with the FMI but negatively with the MF ratio support the notion that the associations of BMI with body composition factors could be diverse.
The impact of the MF ratio on outcome has been previously evaluated in patients with high‐risk factors for sarcopenia, such as advanced CKD.14 By evaluating patients with stage 3–5 CKD not yet treated with dialysis, Lin et al. showed that lean tissue index rather than BMI alone provided better risk prediction of cardiovascular outcome in these patients. Moreover, they found that a high lean/fat tissue index was associated with the best outcomes. Another recent report showed that in patients undergoing maintenance haemodialysis, the mortality risk is increased in those with a high BMI and increased body fat but decreased in those with a high BMI and high muscle mass.32 Despite these previous reports, studies evaluating the effect of body composition on renal outcome in relatively healthy subjects with preserved renal function are lacking. The results of the present study suggest that the balance of fat mass and muscle mass could be a considerable factor when assessing the risk of kidney function decline in the general population.
Several possible mechanisms by which the MF ratio affects kidney function can be postulated. Decrease in muscle mass is frequently linked with chronic inflammation.33 Increased low‐grade systemic inflammation or pro‐inflammatory cytokines related to low muscle mass may consequently induce endothelial dysfunction, leading to a decrease in kidney function.34, 35 In addition, skeletal muscle is the primary site of insulin‐mediated glucose uptake. A previous report has shown that greater muscle mass is clearly associated with increased insulin sensitivity.36 As insulin resistance and related metabolic complications are well‐known factors that damage the kidneys, healthier metabolic features associated with increased muscle mass can contribute to preventing kidney function decline. The circulating levels of fasting glucose, HbA1c, and HOMA‐IR were significantly lower in subjects with a high MF ratio in this study, supporting this possibility. Furthermore, recent studies have suggested that the skeletal muscle is an endocrine organ. Myokines, which are peptides released from the skeletal muscle, have been observed to affect kidney function, a phenomenon proposed as muscle–kidney crosstalk.37 A well‐recognized myokine, irisin, has been demonstrated to improve kidney energy metabolism and prevent kidney damage in mice. On the other hand, increased fat mass is associated with chronic inflammation and induces negative metabolic effects such as impaired glucose tolerance and deranged lipid profile.38 These increased inflammation and negative metabolic effects may lead to structural changes in the kidney, such as mesangial expansion and renal fibrosis.39 Supporting this notion, the present study demonstrated that the levels of CRP, LDL‐C, and triglyceride were significantly higher in subjects with a low MF ratio. In addition, increased fat mass may confer adverse haemodynamic effects on the kidney, inducing higher filtration fraction and increased glomerular capillary pressure.40 Considering these beneficial and adverse properties of muscle and fat mass, the net additive effect of the balance between the two body components could have an impact on kidney function.
This study has several limitations. First, body composition was measured using BIA only. Dual‐energy X‐ray absorptiometry is also considered a reliable method for body composition assessment.41 Nevertheless, previous reports have confirmed that multifrequency BIA systems can provide accurate muscle mass and fat mass values that are comparable with those measured using dual‐energy X‐ray absorptiometry in various populations.42, 43 In addition, the Asian Working Group of Sarcopenia supports the use of BIA for body composition assessment in community‐based settings, owing to its simplicity and reliability.44 Second, a single measurement of body composition at baseline was used for the analysis. As the body composition changes over time, values obtained during a longer follow‐up period may provide a more accurate body composition status. Further investigations considering body composition indices as time‐dependent factors would be needed. Third, evaluations among underweight individuals were not possible due to the small size of this group (120, 1.6%). Further assessments would be needed to verify whether the findings of this study are maintained among those with low BMI. Fourth, although the creatinine levels had been standardized to isotope dilution mass spectrometry reference method values through two different calibration formulae, possibilities of bias still remain. Lastly, owing to the observational study design, a clear causal relationship between a high MF ratio and a lower risk of incident CKD could not be established. Nevertheless, a high MF ratio was independently associated with an increased risk of CKD development even after adjustments for extensive covariates, lowering the chance of bias.
In conclusion, a high MF ratio is associated with a decreased risk of incident CKD in subjects with normal kidney function. This association is more prominent in overweight‐to‐obese subjects. Therefore, in addition to body mass, the balance between body composition components would need to be considered when stratifying the risk of CKD development.
Author contributions
J.J.H. and J.T.P. researched data. J.J.H. wrote the manuscript and researched data. J.T.P. reviewed and edited the manuscript. Y.S.J. contributed to the discussion and reviewed and edited the manuscript. S.D.H., S.H.H., T.H.Y., J.H.S., S.W.L., and S.W.K. researched data and contributed to the discussion. J.T.P. is the guarantor for this work and takes responsibility for the integrity of the data. All authors critically revised the manuscript for key intellectual content and approved the final version of the manuscript. The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle.45
Conflict of interest
None declared.
Supporting information
Table S1. Risk of CKD development according to baseline characteristics
Table S2. Baseline characteristics (Male vs. Female)
Table S3. Univariable correlations between components of body composition and baseline characteristics
Table S4. Baseline characteristics according to sex‐specific median of the MF‐ratio after propensity score matching
Table S5. Risk of CKD development according to body composition indices after propensity score matching
Table S6.Sensitivity analysis: Risk of CKD development according to body composition indices using different creatinine conversion formula
Figure S1.Study subjects
Figure S2.Mutual relationships among components of body composition
Figure S3. Restricted cubic spline plot for incident CKD according to MF‐ratio
Figure S4. Cumulative Hazards for the incident CKD development according to sex‐specific median of the MF‐ratio in all study subjects (A), normal BMI group (B), overweight group (C), and obese group (D)
Figure S5. Subgroup analyses of risk for incident CKD according to high vs. low MF‐ratio groups
Figure S6.Frequency of cumulative study visit months
Acknowledgements
This research was supported by a grant from the Ministry for Health and Welfare, Republic of Korea. The epidemiologic data used in this study were obtained from the Korean Genome and Epidemiology Study (KoGES, 4851‐302) of the Korea Centers for Disease Control and Prevention, Republic of Korea.
Jhee J. H., Joo Y. S., Han S. H., Yoo T.‐H., Kang S.‐W., and Park J. T. (2020) High muscle‐to‐fat ratio is associated with lower risk of chronic kidney disease development, Journal of Cachexia, Sarcopenia and Muscle, 11, 726–734. 10.1002/jcsm.12549.
References
- 1. Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body‐mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014;383:970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. F. H . Obesity Epidemiology. New York: Oxford University Press, Inc.; 2008. [Google Scholar]
- 3. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard‐Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006;355:763–778. [DOI] [PubMed] [Google Scholar]
- 4. Garofalo C, Borrelli S, Minutolo R, Chiodini P, De Nicola L, Conte G. A systematic review and meta‐analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int 2017;91:1224–1235. [DOI] [PubMed] [Google Scholar]
- 5. Brown RN, Mohsen A, Green D, Hoefield RA, Summers LK, Middleton RJ, et al. Body mass index has no effect on rate of progression of chronic kidney disease in non‐diabetic subjects. Nephrol Dial Transplant 2012;27:2776–2780. [DOI] [PubMed] [Google Scholar]
- 6. Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis 2008;52:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prentice AM, Jebb SA. Beyond body mass index. Obes Rev 2001;2:141–147. [DOI] [PubMed] [Google Scholar]
- 8. Okorodudu DO, Jumean MF, Montori VM, Romero‐Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta‐analysis. Int J Obes (Lond) 2010;34:791–799. [DOI] [PubMed] [Google Scholar]
- 9. Harris TB. Invited commentary: body composition in studies of aging: new opportunities to better understand health risks associated with weight. Am J Epidemiol 2002;156:122–124, discussion 125‐126. [DOI] [PubMed] [Google Scholar]
- 10. Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab 2000;279:E366–E375. [DOI] [PubMed] [Google Scholar]
- 11. Son JW, Lee SS, Kim SR, Yoo SJ, Cha BY, Son HY, et al. Low muscle mass and risk of type 2 diabetes in middle‐aged and older adults: findings from the KoGES. Diabetologia 2017;60:865–872. [DOI] [PubMed] [Google Scholar]
- 12. Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009‐2010. Endocr J 2014;61:61–70. [DOI] [PubMed] [Google Scholar]
- 13. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 2011;96:2898–2903. [DOI] [PubMed] [Google Scholar]
- 14. Lin TY, Peng CH, Hung SC, Tarng DC. Body composition is associated with clinical outcomes in patients with non‐dialysis‐dependent chronic kidney disease. Kidney Int 2018;93:733–740. [DOI] [PubMed] [Google Scholar]
- 15. Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, et al. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol 2014;64:997–1002. [DOI] [PubMed] [Google Scholar]
- 16. Srikanthan P, Horwich TB, Tseng CH. Relation of muscle mass and fat mass to cardiovascular disease mortality. Am J Cardiol 2016;117:1355–1360. [DOI] [PubMed] [Google Scholar]
- 17. Lavie CJ, De Schutter A, Patel DA, Romero‐Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the “obesity paradox”. J Am Coll Cardiol 2012;60:1374–1380. [DOI] [PubMed] [Google Scholar]
- 18. Chung JY, Kang HT, Lee DC, Lee HR, Lee YJ. Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Arch Gerontol Geriatr 2013;56:270–278. [DOI] [PubMed] [Google Scholar]
- 19. Graf CE, Karsegard VL, Spoerri A, Makhlouf AM, Ho S, Herrmann FR, et al. Body composition and all‐cause mortality in subjects older than 65 y. Am J Clin Nutr 2015;101:760–767. [DOI] [PubMed] [Google Scholar]
- 20. Ziolkowski SL, Long J, Baker JF, Chertow GM, Leonard MB. Relative sarcopenia and mortality and the modifying effects of chronic kidney disease and adiposity. J Cachexia Sarcopenia Muscle 2019; 10.1002/jcsm.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trajanoska K, Schoufour JD, Darweesh SK, Benz E, Medina‐Gomez C, Alferink LJ, et al. Sarcopenia and its clinical correlates in the general population: the Rotterdam Study. J Bone Miner Res 2018;33:1209–1218. [DOI] [PubMed] [Google Scholar]
- 22. Kim Y, Han BG. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol 2016; 10.1093/ije/dyv316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, et al. Comparison of risk prediction using the CKD‐EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012;307:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee C, Yun HR, Joo YS, Lee S, Kim J, Nam KH, et al. Framingham risk score and risk of incident chronic kidney disease: a community‐based prospective cohort study. Kidney Res Clin Pract 2019;38:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ClinCalc.com . IDMS to conventional serum creatinine [Internet]. ClinCalc LLC [cited 2008. Jun 12]: Available from: https://clincalc . Com/kinetics/idms. Aspx.
- 27. Chang Y, Ryu S, Choi Y, Zhang Y, Cho J, Kwon MJ, et al. Metabolically healthy obesity and development of chronic kidney disease: a cohort study. Ann Intern Med 2016;164:305–312. [DOI] [PubMed] [Google Scholar]
- 28. Jung CH, Lee MJ, Kang YM, Hwang JY, Kim EH, Park JY, et al. The risk of chronic kidney disease in a metabolically healthy obese population. Kidney Int 2015;88:843–850. [DOI] [PubMed] [Google Scholar]
- 29. Lin L, Peng K, Du R, Huang X, Lu J, Xu Y, et al. Metabolically healthy obesity and incident chronic kidney disease: the role of systemic inflammation in a prospective study. Obesity (Silver Spring) 2017;25:634–641. [DOI] [PubMed] [Google Scholar]
- 30. Hashimoto Y, Tanaka M, Okada H, Senmaru T, Hamaguchi M, Asano M, et al. Metabolically healthy obesity and risk of incident CKD. Clin J Am Soc Nephrol 2015;10:578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee MJ, Shin DH, Kim SJ, Oh HJ, Yoo DE, Kim JK, et al. Visceral fat thickness is associated with carotid atherosclerosis in peritoneal dialysis patients. Obesity (Silver Spring) 2012;20:1301–1307. [DOI] [PubMed] [Google Scholar]
- 32. Marcelli D, Usvyat LA, Kotanko P, Bayh I, Canaud B, Etter M, et al. Body composition and survival in dialysis patients: results from an international cohort study. Clin J Am Soc Nephrol 2015;10:1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 2010;91:1128s–1132s. [DOI] [PubMed] [Google Scholar]
- 34. Zoccali C. Endothelial dysfunction and the kidney: emerging risk factors for renal insufficiency and cardiovascular outcomes in essential hypertension. J Am Soc Nephrol 2006;17:S61–S63. [DOI] [PubMed] [Google Scholar]
- 35. Lim SY, Lee KB, Kim H, Hyun YY. Low skeletal muscle mass predicts incident dipstick albuminuria in Korean adults without chronic kidney disease: a prospective cohort study. Nephron 2018; 10.1159/000494392:1-7. [DOI] [PubMed] [Google Scholar]
- 36. De Cosmo S, Menzaghi C, Prudente S, Trischitta V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant 2013;28:29–36. [DOI] [PubMed] [Google Scholar]
- 37. Peng H, Wang Q, Lou T, Qin J, Jung S, Shetty V, et al. Myokine mediated muscle‐kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat Commun 2017;8:1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Madero M, Katz R, Murphy R, Newman A, Patel K, Ix J, et al. Comparison between different measures of body fat with kidney function decline and incident CKD. Clin J Am Soc Nephrol 2017;12:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anderson PW, Zhang XY, Tian J, Correale JD, Xi XP, Yang D, et al. Insulin and angiotensin II are additive in stimulating TGF‐β1 and matrix mRNAs in mesangial cells. Kidney Int 1996;50:745–753. [DOI] [PubMed] [Google Scholar]
- 40. Ribstein J, du Cailar G, Mimran A. Combined renal effects of overweight and hypertension. Hypertension 1995;26:610–615. [DOI] [PubMed] [Google Scholar]
- 41. Prior BM, Cureton KJ, Modlesky CM, Evans EM, Sloniger MA, Saunders M, et al. In vivo validation of whole body composition estimates from dual‐energy X‐ray absorptiometry. J Appl Physiol (1985) 1997;83:623–630. [DOI] [PubMed] [Google Scholar]
- 42. Boneva‐Asiova Z, Boyanov MA. Body composition analysis by leg‐to‐leg bioelectrical impedance and dual‐energy X‐ray absorptiometry in non‐obese and obese individuals. Diabetes Obes Metab 2008;10:1012–1018. [DOI] [PubMed] [Google Scholar]
- 43. Stewart SP, Bramley PN, Heighton R, Green JH, Horsman A, Losowsky MS, et al. Estimation of body composition from bioelectrical impedance of body segments: comparison with dual‐energy X‐ray absorptiometry. Br J Nutr 1993;69:645–655. [DOI] [PubMed] [Google Scholar]
- 44. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 45. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Risk of CKD development according to baseline characteristics
Table S2. Baseline characteristics (Male vs. Female)
Table S3. Univariable correlations between components of body composition and baseline characteristics
Table S4. Baseline characteristics according to sex‐specific median of the MF‐ratio after propensity score matching
Table S5. Risk of CKD development according to body composition indices after propensity score matching
Table S6.Sensitivity analysis: Risk of CKD development according to body composition indices using different creatinine conversion formula
Figure S1.Study subjects
Figure S2.Mutual relationships among components of body composition
Figure S3. Restricted cubic spline plot for incident CKD according to MF‐ratio
Figure S4. Cumulative Hazards for the incident CKD development according to sex‐specific median of the MF‐ratio in all study subjects (A), normal BMI group (B), overweight group (C), and obese group (D)
Figure S5. Subgroup analyses of risk for incident CKD according to high vs. low MF‐ratio groups
Figure S6.Frequency of cumulative study visit months


