Figure 1.
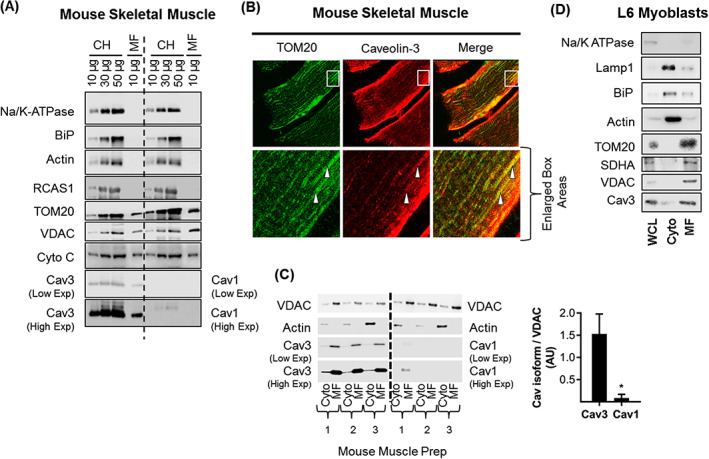
Cav3 is closely associated with mitochondria in mouse skeletal muscle and rat L6 myoblasts. Crude muscle homogenates (CH, 10, 30, 50 μg of protein) and isolated mitochondrial fractions (MF, 10 μg) from mouse gastrocnemius were used for sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotting using antibodies to the proteins indicated (A). Mouse gastrocnemius was sectioned and stained for mitochondrial marker TOM20 and Cav3. The field within the white box has been enlarged to highlight potential co‐localization (indicated by the white arrowheads) of TOM20 and Cav3 (B). Mitochondrial (MF, 10 μg protein) and cytosolic (Cyto, 10 μg protein) fractions isolated from gastrocnemius muscle of three separate mice were immunoblotted with antibodies to proteins indicated. Mitochondrial abundance of Cav1 and Cav3 were quantified (using the low exposure blots) relative to that of VDAC (C). Whole cell lysates (WCL, 5 μg protein), cytosolic (cyto, 5 μg protein) and mitochondrial fractions (MF, 5 μg protein) prepared from L6 myoblasts were subject to sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted with antibodies to proteins indicated (D). The blots shown in (D) are representative of three separate experimental preparations.The graphical data represent mean ± SEM from a minimum of three separate experiments. Asterisks indicate a significant change (P < 0.05). Cav3, caveolin‐3; SDHA, succinate dehydrogenase subunit A; VDAC, voltage‐dependent anion channel.
