Figure 3.
N-Cadherin/β-Catenin Complex Associates with the C-Terminal Domain of LiGluK2
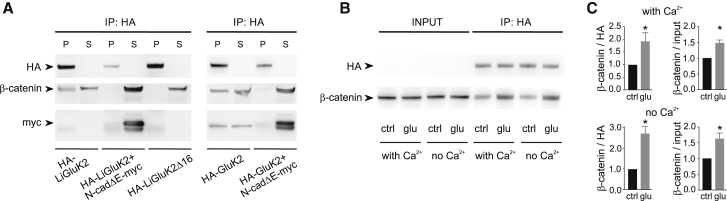
(A) Left: coimmunoprecipitation experiment showing the interaction between HA-LiGluK2 and the β-catenin/N-cadherin complex. Cell lysates from HEK293 cells transfected with Li-GluK2 alone, LiGluK2 along with N-cadΔE-myc, or HA-LiGluK2Δ16 were immunoprecipitated with the anti-HA antibody and immunoblotted with anti-HA, anti-β-catenin, and anti-myc antibodies. β-catenin co-precipitates with HA-LiGluK2 transfected alone, but not with HA-LiGluK2 cotransfected along with the dominant-negative N-cadΔE-myc. β-catenin fails to interact with the mutant receptor HA-LiGluK2Δ16. Right: coimmunoprecipitation experiment showing the interaction between HA-GluK2 and the β-catenin/N-cadherin complex. Cell lysates from HEK293 cells transfected with GluK2 alone or GluK2 along with N-cadΔE-myc were immunoprecipitated and immunoblotted. β-catenin co-precipitates with HA-GluK2, similarly to full-length HA-LiGluK2. The dominant-negative N-cadΔE-myc prevents the interaction of β-catenin with HA-GluK2. Note that the presence of N-cadΔE-myc in the supernatants (S) indicates that it does not interact with full-length LiGluK2 and GluK2 receptors.
(B) Glutamate enhances the coprecipitation of HA-LiGluK2 with β-catenin. Anti-HA immunoprecipitation (IP) in the control condition (ctrl) and upon receptor desensitization with glutamate 100 μM (glu) in the presence or absence of calcium. The “input” represents 10% of the total extract before the IP.
(C) Quantification of the co-precipitation experiments described in (B). Effect of glutamate on β-catenin co-IP with LiGluK2 in the presence (top) or absence (bottom) of calcium. Bar plots represent IP β-catenin signal normalized to HA-LiGluK2 signal (left) or to β-catenin input (right). n = 6.
Data are presented as mean ± SEM, ∗p < 0.05.