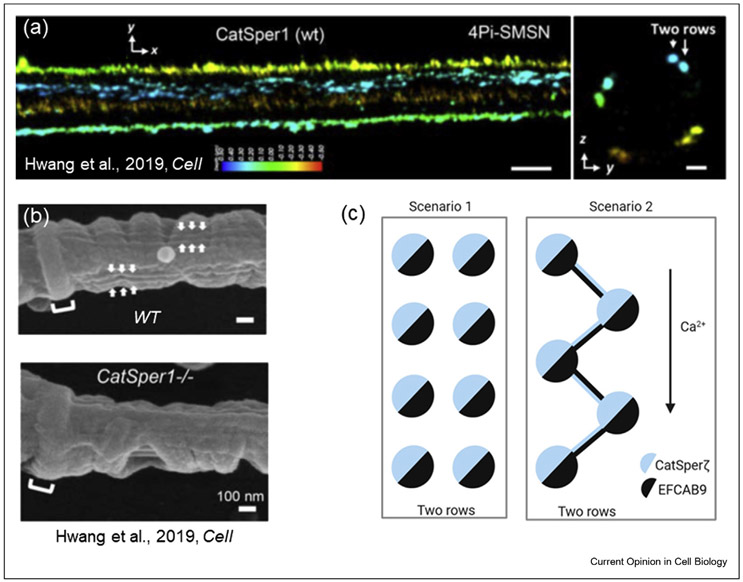
Figure 2. Two possible flagellar arrangements of CatSper nanodomains.
(a) Reproduced from Ref. [••42]: 4Pi single-molecule switching nanoscopy images of murine CatSper1 in wild type (WT) flagella. x–y projection colors encode the relative distance from the focal plane along the z axis. Scale bar, 500 nm. Right panel: y–z cross sections (100 nm thick). Two-row structures are indicated with arrows. Scale bar, 200 nm. (b) SEM images of the principal piece of flagella from WT (top panel) and CatSper1−/− (bottom panel) mice. As indicated by arrows, double-row lines are observed on both sides of WT flagellum within each longitudinal column. These structures are absent in CatSper1−/− flagellum. Scale bar, 100 nm. (a) and (b) figures are from Ref. [••42]. (c) Two possible scenarios of CatSper flagellar nanodomain architecture. As shown in (a), CatSper forms two parallel rows and as suggested by Hwang et al. [••42], CatSper-zeta associates with EFCAB9 within the same CatSper complex (left panel). According to an alternative scenario (right panel), CatSper-zeta and EFCAB9 link together two neighboring channels in a zig-zag manner, in which CatSper-zeta from one CatSper complex is associated with EFCAB9 from the neighboring CatSper complex. This hypothetical arrangement would not only structurally link CatSper complexes, but also link them functionally. This linkage could permit more efficient signal propagation along the flagellar length and could be responsible for either synchronization of CatSper opening or longitudinal propagation of Ca2+ waves.