Abstract
In the current investigation, the active principles of the methanol extracts of Rhododendron arboreum leaves (MEL) and flowers (MEF) were investigated with the help of ultra-high performance liquid chromatography (UHPLC), amino acid analyzer and gas chromatography mass spectrometry (GC-MS). UHPLC revealed different polyphenols present in the extracts. GC-MS identified 20 phytochemicals in leaves and 17 in the flowers, whereas, amino acid analyzer confirmed 11 amino acids in leaves and 10 in the flowers. The extracts were subjected to the investigation of biological activity through analysis of antioxidant activity in different in vitro assays, antimutagenic activity in Ames assay and cancer cell growth inhibition activity by MTT (3-4,5 dimethylthiazol-2,5 diphenyltetrazolium bromide) assay. MEL showed higher antioxidant activity in lipid peroxidation inhibition assay (95.32 ± 0.37%) than MEF (77.09 ± 4.17%) with IC50103.6 µg/ml for MEL and 271.17 µg/ml for MEF. In nitric oxide scavenging assay, an activity of 94.46 ± 0.32% (IC50 150.13) was observed in MEF followed by 83.71 ± 0.74% (IC50 179.52) in MEL. The antimutagenic activity of both the extracts was evaluated against sodium azide, 4-nitro-O-phenylenediamine and 2-aminofluorene mutagens in TA-98 and TA-100 strains of Salmonella typhimurium. The analysis was carried out using pre- and co-incubation modes. However, both extracts were observed to possess considerable antimutagenic activity against different known mutagens, flowers came out to be more effective than the leaves in terms of % inhibition. The extracts also exhibited significant cancer cell growth inhibition activity, when tested against 3 cancer cell lines namely, Human cervical cancer cell line (HeLa), Breast cancer cell line (MCF7) and Lung cancer cell line (A549). In case of HeLa and A549, MEL showed higher activity of 64.62 ± 2.65 and 75.08 ± 1.68% as compared to 53.11 ± 2.84 and 45.92 ± 2.43% in MEL, respectively. The EC50 values for MEL in HeLa and A549 were noted to be 232.76 and 155.38 µg/ml, respectively, whereas, MEF had EC50 of 395.50 µg/ml in HeLa and 660.26 µg/ml in A549. Further, MEF showed higher cytotoxicity in MCF7 cell line (84.93 ± 1.17%) followed by the MEL (73.57 ± 1.27%) with EC50 value of 95.16 µg/ml for MEF followed by 172.19 µg/ml for MEL. The biological activities of the extracts can be attributed to the phyto-constituents identified by sophisticated instruments.
The biological activities of the extracts can be attributed to the active principles identified by sophisticated instruments.
Keywords: Antimutagenic activity, Antioxidant activity, Rhododendron arboretum
1. Introduction
Oxygen is essential for life as it creates the energy required to carry on the functioning of cells through the process of oxidation. The oxidation results in the formation of various reactive oxygen species (ROS). In plants majority of ROS are produced in the respiratory and photosynthetic electron transport chains (Racchi, 2013) and are involved in various other biological processes such as gene expression and mediation of redox reactions (Suzuki et al., 1997). Meanwhile, free radicals have potential to damage the cells and cellular structures like nucleic acids, lipids, carbohydrates and proteins and therefore, change their utility (Birben et al., 2012). This either directly leads to different diseases e.g. neurological disorders, early aging, Alzheimer’s disease, atherosclerosis, gastritis, cardiovascular diseases, diabetes, or cause mutations in DNA, which is further a cause if several dreadful diseases including cancer (Valko et al., 2007, Hela and Abdullah, 2010).
The plant derived phytochemicals which have high antioxidant, antimutagenic and anticancer activities and low toxicity to mammals and possess medicinal properties to maintain human health, have attained special attention of researchers in recent decades. Researchers believe that the oxidative stress and its concequences such as DNA mutations and cancer development can be decreased by consuming antioxidant rich foods containing phenolic compounds and many other phytochemicals that have antioxidant properties (Smith et al., 1996, Markesbery, 1997, Weinbrenner et al., 2003). To conquer the oxidative stress and to uphold the optimum intensity of ROS, various antioxidants are recommended as essential dietary intake. The dietary intake of antioxidants from external sources minimizes the risk of mutations, cancer and other hundreds of diseases caused by ROS. The synthetic antioxidants impose chronic toxicity and side effects on health. Therefore, the interest of researchers is growing in identifying and isolating the natural antioxidants from the plants. The plants, especially the angiosperms are rich in polyphenols and other compounds that are proved antioxidants (Hagerman et al., 1998). Polyphenols act as free-radical scavengers and powerful anti-oxidants (Bennick, 2002). These natural antioxidants are also byproducts of nature and are considered as non-toxic and have no side-effects.
Rhododendron arboreum, an angiosperm bearing eye catchy bright red flowers, belongs to family Ericaceae. The name ‘rhododendron’ is derived from the Greek word ‘rhodo’ means rose & ‘dendron’ means tree. It is native to Bhutan, China, India and Nepal. In India, it is abundantly found in the high altitudes of North and North-East India. R. arboreum is the national flower of Nepal and the state tree of Uttarakhand. This evergreen tree is significant from economical as well as horticultural viewpoint. Also, it is widely used by tribal people of North India for cooking as well as traditional curative purposes. R. arboreum flowers are used for making jellies, local brew and jams in hilly areas of Himachal Pradesh (Bhattacharjee, 1998). The fresh flowers of R. arboreum are used in treatment of dysentery and diarrhea whereas dried flowers are taken to cure blood dysentery (Bhattacharjee, 1998, Laloo et al., 2006). R. arboreum is reported to be effective as diuretic, choleretic, chronic diarrhea, anti-irritable bowel syndrome therapy and astringent (Matin et al., 2001). Young leaves of this species are poisonous and are applied on forehead alleviating headache (Watt, 1982). Different parts of Rhododendron have been reported to possess a wide range of pharmacological activities, such as anti-oxidant, anti-inflammatory, anti-nociceptive, anti-diabetic, anti-diarrheal and hepatoprotective (Kemertelidze et al., 2007, Shyam and Kalpana, 1988, Gautam et al., 2018). It has also been reported to be a resource of a number of phytoconstituents of therapeutic value by numerous authors (Painuli et al., 2016, Roy et al., 2014, Kiruba et al., 2011). However, R. arboreum is widely used in conventional therapeutic practices but there are not many scientific reports to confirm its antimutagenic and anticancer properties. Therefore, in current study we have selected the leaves and flowers of R. arboreum and evaluated the biological activities of methanol extracts of leaves and flowers through antioxidant activity in different in vitro assays, antimutagenic activity with the Ames assay, cancer cell growth inhibition activity with the MTT assay, polyphenols contents using UHPLC, phytochemicals using GC-MS and amino acid analysis using amino acid analyzer.
2. Materials and methods
2.1. Sample preparation
The leaves and flowers of Rhododendron arboreum were procured from Kataula village in the Kullu district of Himachal Pradesh in the month of March and were identified and authenticated at the herbarium of Guru Nanak Dev University, Amritsar. The leaves and flowers were washed with double distilled water, dried in shade, and ground to fine powder separately in a mixer–grinder. The 1 kg leaf powder was then extracted with 80% methanol. The extractant was dried out with the help of rotary vacuum evaporator at a temperature of 30 °C to get 68.38 g (6.83%) methanol extract of leaves (MEL), whereas, 1 kg flower powder was also extracted with 80% methanol and dried in rotary vacuum evaporator at 30 °C to get 72.12 g (7.21%) methanol extract of flower (MEF). At first, 1000 µg/mL stock solutions of MEL and MEF were prepared, which were further used to make different concentrations for different assays using serial dilution. For antioxidant assays, 200, 400, 600, 800, 1000 µg/mL concentrations were prepared, whereas, to assess the antimutagenic activity, 100, 500, 1000 and 2500 µg/mL and for cancer cell growth inhibition activity, 30, 50, 100, 500 µg/mL concentrations of MEL and MEF were prepared. All the chemicals used in extraction as well as experimentation were analytical grade and procured from Sigma-Aldrich. For phytochemical (UHPLC, GC-MS and amino acid analyzer) analysis, HPLC grade chemicals obtained from Sigma-Aldrich were used. All solutions were prepared with deionised water.
2.2. Polyphenol estimation
Qualitative as well as quantitative analysis of the plant samples for polyphenolic compounds was carried out using the ultra-high performance liquid chromatography (UHPLC). 500 mg of fresh plant material was crushed in 2 ml of HPLC grade methanol, centrifuged at 13000 rpm for 20 min and filtered using 0.2-µm filter paper. The portrayal of phenolic compounds was executed using 130 MPa Shimadzu UHPLC (Nexera) system. The chromatography was performed following to the similar conditions and procedures used by Gautam et al. (2018). The identification of each compound was based on a combination of retention time and spectral resemblance. The recognition and quantification limit for all the detected compounds were designed on the basis of signal-to-noise ratio (S/N) of 3 and 10 with equivalent standard solution, correspondingly.
2.3. GC-MS analysis
The methanol extracts of flowers and leaves of R. arboreum Sm. were subjected to Shimadzu GCMS-QP2010 Plus system in similar conditions used by Gautam et al. (2018) for identification of different phytochemicals. The discovered compounds were recognized by match up to mass spectra with National Institute of Standard and Technology (NIST08s) and Wiley 7 library.
2.4. Amino acid analysis
One mg of sample was dissolved in 1 ml of methanol. 1 ml of 6% sulfosalicylic acid was mixed with above solution and mixture was further diluted with 0.1 N hydrochloric acid. The solution was filtered using 0.2-µm filter paper. The amino acid analysis was carried out using Shimadzu, Nexera X2 amino acid analyzer. 150 mm long C-18 silica bonded column with 120 Å pore size and 5 µm particle size was used for the analysis of amino acids. Injection volume was 1 µL; run time was 32 min; oven temperature was 40 °C; pump flow rate was 1ml/min. Mobile Phase “A” consisted of 5.6 pH phosphate buffer; Mobile phase “B” comprised of ultra-pure water, methanol and acetonitrile in the ratio of 15:40:45. The recognition of each amino acid was dependent on arrangement of retention time and spectral resemblance.
2.5. Antioxidant activity
2.5.1. Lipid peroxidation inhibition assay
Method of Halliwell and Gutteridge (2015) was used with minor amendments. 1 ml of the different concentrations (200, 400, 600, 800 and 1000 µg/mL) of extracts were combined with 0.5 ml potassium chloride (KCl) and 0.5 ml rat liver homogenate. Addition of 100 µL of ferric chloride (FeCl3) was done induce lipid peroxidation. After an incubation of 30 mins at 37 °C, 2 ml of chilled 0.25 N hydrochloric acid (HCl) containing 15% trichloroacetitic acid (TCA) and 0.5% thiobarbituric acid (TBA) was added to the mixture followed by addition of 50 µL of butylated hydroxytoluene (BHT) and an incubation of 60 min at 100 °C. After incubation, the mixture was centrifuged at 900 rpm for 3 min and the absorbance was noted at 532 nm using spectrophotometer. Gallic acid was used as standard reference compound. The calculation of defending effect of extracts in opposition to lipid peroxidation was done as:
2.5.2. Nitric oxide scavenging assay
Minor modifications were adapted to the method given by Marcocci et al. (1994), to assess the extracts for their nitric oxide scavenging activity. 200, 400, 600, 800 and 1000 µg/mL concentrations of MEL an MEF were used. 0.5 ml of 5 mM sodium nitropruside in 7.3 pH phosphate buffer was mixed with 1 ml of extract followed by 30 min’ incubation at 25 °C. 1.5 ml Griess reagent was added to the above mixture followed by absorbance measurement at 546 nm. In the control, the similar amount of 5 mM phosphate buffer was used in place of plant extract with same reaction mixture. Ascorbic acid was used as standard. The scavenging of nitric oxide was calculated as:
2.6. Antimutagenic activity
The antimutagenic activity of methanol extracts of R. arboreum leaves and flowers was investigated by using the method given by Maron and Ames (1983) with minor changes suggested by Bala and Grover (1989). 4-Nitro-o-phenylenediamine (NPD) and sodium azide were used as direct acting mutagens for TA-98 and TA-100 tester strains of Salmonella typhimurium respectively, whereas, 2-Aminofluorene (2AF) was used as a promutagen for both the strains and S9 rat liver homogenate was used to activate the promutagen by the cytochrome based P450 metabolic activation system (Garner et al., 1972). The 100, 500, 1000, 2500 µg/0.1 ml concentrations of plant extracts were prepared in dimethyl sulfoxide (DMSO) and the analysis was carried out using pre-incubation (PI) and co-incubation (Co-I) modes of experiment so that the bio-antimutagenicity and desmutagenicity could be differentiated. The antimutagenic activity of the extracts was expressed as % decrease of reverse mutations as follows:
2.7. Cancer cell growth inhibition activity
The cytotoxic effect of methanol extracts of R. arboretum leaves and flowers against three cancer cell lines namely; HeLa (human cervical cancer cell line), MCF7 (breast cancer cell line) and A549 (lung cancer cell line) was estimated by MTT (3-4, 5 dimethylthiazol-2, 5 diphenyltetrazolium bromide) assay (Mosmann, 1983). Principle of MTT assay is that water soluble blue coloured MTT dye is converted into formazan by viable cells. Formazan is Purple in colour and insoluble in water upon reduction by mitochondrial oxidoreductase enzymes. As the MTT-formazan is dissolved in an appropriate solvent, the quantity of MTT-formazan produced can be detected with spectrophotometer. The growth of cancer cell lines was allowed up to sub confluent stage by seeding them in culture flask. Then the cells were harvested by trypsinization medium. The cell suspension was centrifuged for five minutes at 1500 rpm and the resultant pellet of cells was mixed with growth medium and further checked for viability by taking cell count with trypan blue dye. 100 µL cell suspension was put in 96 well plate to get 5000 cells per well. The different concentrations (30, 50, 100 and 500 µg/ml DMEM) of extracts of R. arboreum leaves and flowers were prepared by serial dilution using the growth medium in the 96 wells plate. The positive control for all cell lines was camptothecin (cytotoxic quinoline alkaloid). After an incubation of 24 h, 100 µL MTT dye was added in each well and incubated for next 4 to 5 h so that MTT gets reduced to formazan. The medium was discarded and added DMSO (100 μL) in every well so that crystals of formazan get dissolved. The well plate was shaked for 7–8 min and absorbance was noted at 595 nm using ELISA (Biotek Synergy HT) plate reader. Drop in the quantity of living cells leads to decreased formazan crystals formation, thereby, reduction in absorbance. The cytotoxicity was calculated by using the formula:
where ODC = Optical density of control, ODS = Optical density of extract
2.8. Statistical analysis
The results are represented as mean ± standard error of three replications. 1-way and 2-way analysis of variance (ANOVA) was executed for the interactions at 5% level of significance.
3. Results
3.1. Polyphenols
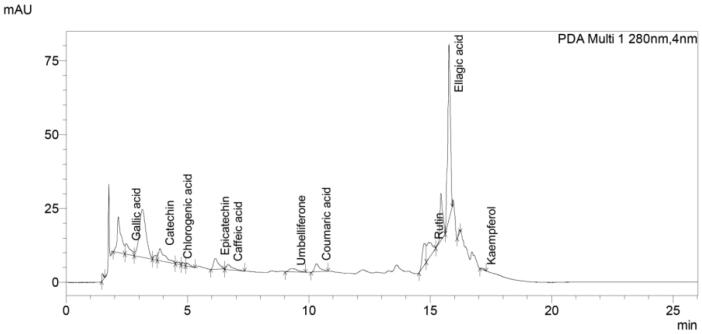
UHPLC study confirmed the presence of eleven polyphenols in the methanolic leaf extract of Rhododendron arboretum Sm. (Fig. 3). Quercetin (C15H10O7) was found to be absent in the MEL. The concentrations of the polyphenols were as follows: gallic acid (3.66 ppm), catechin (32.5 ppm), chlorogenic acid (1.098 ppm), epicatechin (14.08 ppm), caffeic acid (2.01 ppm), umbelliferone (5.75 ppm), coumaric acid (1.39 ppm), rutin (20.51 ppm), ellagic acid (98.003 ppm) and kaempferol (1.56 ppm).
Fig. 3.
UHPLC chromatogram showing the polyphenols in methanol extract of Rhododendron arboreum leaves.
Eleven polyphenols were found to be present in the MEF (Fig. 4). The concentrations of the found polyphenols are: gallic acid (15.06 ppm), catechin (117.49 ppm), chlorogenic acid (110.58 ppm), epicatechin (10.14 ppm), caffeic acid (19.55 ppm), umbelliferone (4.65 ppm), coumaric acid (6.71 ppm), rutin (65.41 ppm), ellagic acid (148.48 ppm), quercetin (1.4 ppm) and kaempferol (8.83 ppm).
Fig. 4.
UHPLC chromatogram showing the polyphenols in methanol extract of Rhododendron arboreum flowers.
3.2. GC-ms
20 phytochemicals in the methanolic leaf extract were identified qualitatively with the help of GCMS analysis (Table 2). Those compounds are: 2-Hexenal, 3-n-Butylthiolane, Quinic acid, [1,1′-Bicyclopropyl]-2-octanoic acid, Dihydro Linalool, 2(3H)-Benzothiazolethione, Benzenepropanoic acid, Hexadecanoic acid, 2-Hexadecen-1-ol, 8,11,14-Eicosatrienoic acid, Linoleic acid, 9-Octadecenoic acid, Farnesol, Heptacosane, Tricyclo[4.3.0.0(7,9)]nonane, Vitamin A aldehyde, Methyl commate D, Methyl commate B, Flavone 4′-OH, 9,12-Octadecadienoic acid.
Table 2.
Phytochemicals detected by GCMS analysis in methanol extract of Rhododendron arboreum Sm. leaves (MEL).
| Sr. No. | Retention Time | Area | Area% | Height | Name |
|---|---|---|---|---|---|
| 1 | 4.48 | 378,827 | 1.21 | 91,335 | 2-Hexenal |
| 2 | 5.92 | 434,531 | 1.39 | 90,562 | 3-n-Butylthiolane |
| 3 | 15.4 | 2,948,501 | 9.41 | 291,894 | Quinic acid |
| 4 | 17.63 | 306,438 | 0.98 | 215,396 | [1,1′-Bicyclopropyl]-2-octanoic acid |
| 5 | 17.88 | 62,471 | 0.20 | 43,877 | Dihydro Linalool |
| 6 | 17.99 | 278,471 | 0.89 | 159,662 | 2(3H)-Benzothiazolethione |
| 7 | 18.36 | 989,117 | 3.16 | 736,597 | Benzenepropanoic acid |
| 8 | 18.81 | 937,130 | 2.99 | 473,407 | Hexadecanoic acid (CAS) Palmitic acid |
| 9 | 20.24 | 302,323 | 0.97 | 180,451 | 2-Hexadecen-1-ol |
| 10 | 20.39 | 146,902 | 0.47 | 95,326 | 8,11,14-Eicosatrienoic acid |
| 11 | 20.43 | 119,914 | 0.38 | 85,200 | Linoleic acid |
| 12 | 20.68 | 242,362 | 0.77 | 115,313 | 9-Octadecenoic acid |
| 13 | 26.56 | 154,866 | 0.49 | 68,782 | Farnesol |
| 14 | 27.74 | 1,214,844 | 3.88 | 465,960 | Heptacosane |
| 15 | 33.53 | 1,621,595 | 5.18 | 297,985 | Tricyclo[4.3.0.0(7,9)]nonane |
| 16 | 35.06 | 983,814 | 3.14 | 178,742 | Vitamin A aldehyde |
| 17 | 35.32 | 2,970,360 | 9.48 | 671,110 | Methyl commate D |
| 18 | 36.13 | 12,105,509 | 38.65 | 1,707,855 | Methyl commate B |
| 19 | 37.81 | 4,419,213 | 14.11 | 1,476,326 | Flavone 4′-OH |
| 20 | 39.85 | 707,302 | 2.26 | 164,099 | 9,12-Octadecadienoic acid |
Methanol extract of R. arboreum flowers revealed the presence of 17 different phytochemicals in GCMS analysis (Table 3). These phytochemicals are 4H-pyran-4-one, 2-furancarboxaldehyde, benzenepropanoic acid, 9,12-octadecadienoic acid, 9-octadecenoic acid, docosanoic acid, linoleic acid, hexadecanoic acid, [1,1′-bicyclopropyl]-2-octanoic acid, D:A-friedooleanan-28-al, beta-sitosterol, methyl commate E, acetic acid, retinal, hexadecanoic acid, flavone 4′-OH and agathic acid.
Table 3.
Phytochemicals detected by GCMS analysis in methanol extract of Rhododendron arboreum Sm. flowers (MEF).
| Sr. No. | Retention Time | Area | Area% | Height | Name |
|---|---|---|---|---|---|
| 1 | 6.06 | 6,728,849 | 5.22 | 924,387 | 4H-Pyran-4-one |
| 2 | 8.57 | 43,372,507 | 33.64 | 3,244,252 | 2-Furancarboxaldehyde |
| 3 | 18.38 | 4,137,875 | 3.21 | 2,956,330 | Benzenepropanoic acid |
| 4 | 18.89 | 14,167,936 | 10.99 | 3,786,744 | Hexadecanoic acid (CAS) Palmitic acid |
| 5 | 20.45 | 23,772,750 | 18.44 | 3,766,841 | 9,12-Octadecadienoic acid |
| 6 | 20.73 | 2,771,534 | 2.15 | 1,605,660 | 9-Octadecenoic acid |
| 7 | 22.45 | 657,472 | 0.51 | 439,518 | Docosanoic acid |
| 8 | 22.96 | 881,103 | 0.68 | 365,386 | Linoleic acid |
| 9 | 23.46 | 771,494 | 0.60 | 350,027 | Hexadecanoic acid, 2,3-dihydroxypropyl ester |
| 10 | 25.74 | 494,159 | 0.38 | 248,535 | [1,1′-Bicyclopropyl]-2-octanoic acid |
| 11 | 33.54 | 1,539,426 | 1.19 | 300,071 | D:A-Friedooleanan-28-al |
| 12 | 35.15 | 17,359,344 | 13.46 | 3,093,659 | beta.-Sitosterol |
| 13 | 35.35 | 2,102,077 | 1.63 | 448,764 | Methyl commate E |
| 14 | 36.07 | 3,381,240 | 2.62 | 529,241 | Acetic acid |
| 15 | 37.19 | 1,316,202 | 1.02 | 413,572 | Retinal |
| 16 | 37.81 | 2,628,220 | 2.04 | 932,297 | Flavone 4′-OH |
| 17 | 39.5 | 2,864,221 | 2.22 | 948,283 | Agathic acid |
3.3. Amino acids
A qualitative as well as quantitative analysis of amino acids was carried out using amino acid analyzer. MEL showed the presence of 11 amino acids (Table 4). Names and quantities of those are: Aspartic Acid (7.87 ppm), Glutamic acid (1.73 ppm), Serine (1.5 ppm), Glutamine (14.58 ppm), Citrulline (2.26 ppm), Alanine (4.78 ppm), Cystine (1.04 ppm), Isoleucine (1.2 ppm), Leucine (9.62 ppm), Lysine (1.78 ppm), Proline (5.56 ppm). Threonine, B-Alanine, Tyrosine and Tryphtophan were not detected in MEL.
Table 4.
Different Amino acids present in methanol extracts of Rhododendron arboreum leaves (MEL) and flowers (MEF).
| Amino Acid | Concentration (ppm) |
|
|---|---|---|
| MEL | MEF | |
| Aspartic Acid | 7.87 | 43.74 |
| Glutamic acid | 1.73 | 2.68 |
| Serine | 1.5 | 1.6 |
| Glutamine | 14.58 | 27.58 |
| Citrulline | 2.26 | – |
| Threonine | – | 3.59 |
| Alanine | 4.78 | 4.83 |
| B-Alanine | – | – |
| Tyrosine | – | 3.43 |
| Cystine | 1.04 | 7.32 |
| Tryphtophan | – | – |
| Isoleucine | 1.2 | – |
| Leucine | 9.62 | – |
| Lysine | 1.78 | 0.6 |
| Proline | 5.56 | 7.65 |
The amino acid analysis revealed the presence of 10 amino acids in MEF (Table 4). These amino acids were: aspartic acid (43.74 ppm), glutamic acid (2.68 ppm), serine (1.6 ppm), glutamine (27.58 ppm), threonine (3.59 ppm), alanine (4.83 ppm), tyrosine (3.43 ppm), cystine (7.32 ppm), lysine (0.6 ppm), proline (7.65 ppm). Five amino acids namely, citrulline, B-alanine, tryphtophan, isoleucine and leucine were not detected in methanol extract of R. arboreum flowers.
3.4. Antioxidant activity
3.4.1. Lipid peroxidation
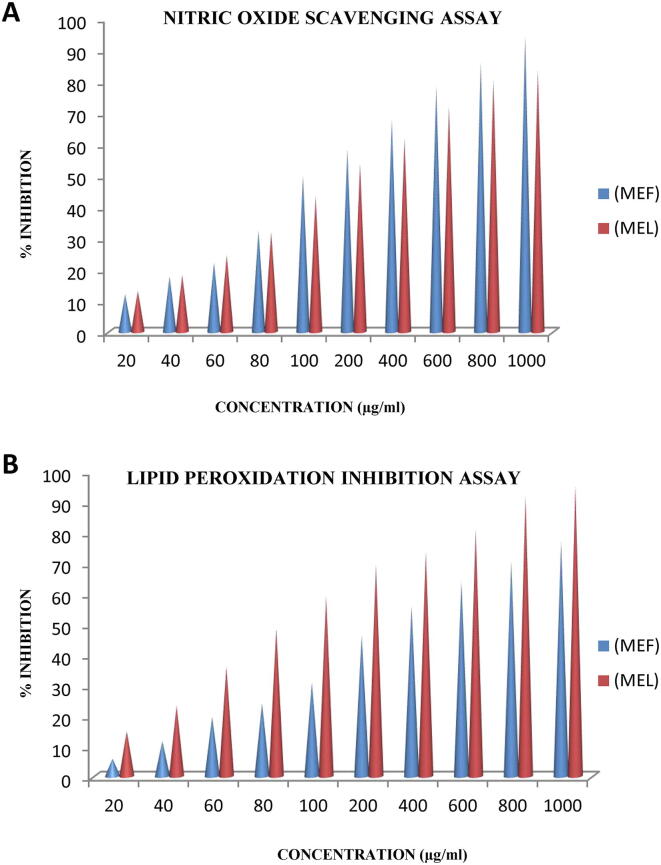
In lipid peroxidation inhibition activity, MEL showed 14.97 ± 2.52% inhibition at 20 µg/ml concentration. The activity was noted to be 95.32 ± 0.37% at the maximum tested concentration (1000 µg/ml). IC50 value was 103.6 µg/ml. The lipid peroxidation inhibition activity of MEF is depicted in Fig. 1. The extract showed 5.80 ± 0.94% inhibition at 20 µg/ml concentration. With an increase of 12.28 folds, the activity was noted to be 77.09 ± 4.17% at a concentration of 1000 µg/ml. IC50 value was calculated to be 271.17 µg/ml.
Fig. 1.
Antioxidant activity of different concentrations of methanol extracts of Rhododendron arboreum leaves (MEL) and flowers (MEF) in (A) nitric oxide scavenging assay and (B) lipid peroxidation assay.
3.4.2. Nitric oxide scavenging
Nitric oxide radical scavenging activity of both the extracts was enhanced with increasing concentrations (Fig. 1). At lowest concentration (20 µg/ml) the activity of MEL was 13.20 ± 1.44% whereas at highest applied concentration (1000 µg/ml), the activity was 83.71 ± 0.74%. Fold increase in activity was 5.34. The IC50 was calculated to be 179.52 µg/ml (Table 1). In case of MEF, at lowest tested concentration (20 µg/ml) the activity was 12.09 ± 1.03%, whereas, at highest tested concentration (1000 µg/ml), the activity was 94.46 ± 0.32%. Fold increase in activity was 6.81. The IC50 was calculated to be 150.13 µg/ml.
Table 1.
Growth inhibitory effects of methanol extracts of Rhododendron arboreum leaves (MEL) and flowers (MEF) on different cancer cells in MTT assay.
| Concentration (µg/ml) | % Growth Inhibition |
|||||
|---|---|---|---|---|---|---|
| HeLa |
MCF7 |
A549 |
||||
| MEL | MEF | MEL | MEF | MEL | MEF | |
| 30 | 17.04 ± 0.57 | 8.29 ± 1.76 | 15.45 ± 0.54 | 19.04 ± 1.43 | 17.73 ± 0.77 | 13.05 ± 1.58 |
| 50 | 23.59 ± 0.92 | 16.23 ± 0.64 | 18.98 ± 0.75 | 30.63 ± 0.8 | 22.48 ± 1.29 | 20.46 ± 1.03 |
| 100 | 32.09 ± 0.75 | 27.27 ± 1.26 | 38.87 ± 2.57 | 54.01 ± 2.02 | 41.31 ± 1.24 | 30.28 ± 1.02 |
| 500 | 64.62 ± 2.65 | 53.11 ± 2.84 | 73.57 ± 1.27 | 84.93 ± 1.17 | 75.08 ± 1.68 | 45.92 ± 2.43 |
| EC-50 | 232.76 | 395.50 | 172.19 | 95.16 | 155.38 | 660.26 |
| F Ratio (3,8) | 608.64*** | 381.9*** | 934.03*** | 1241.07*** | 1221.00*** | 229.19*** |
| HSD | 3.87 | 5.82 | 3.94 | 3.73 | 3.37 | 4.25 |
Values expressed as Mean ± SE; *** significance at p ≤ 0.001
3.5. Antimutagenic activity
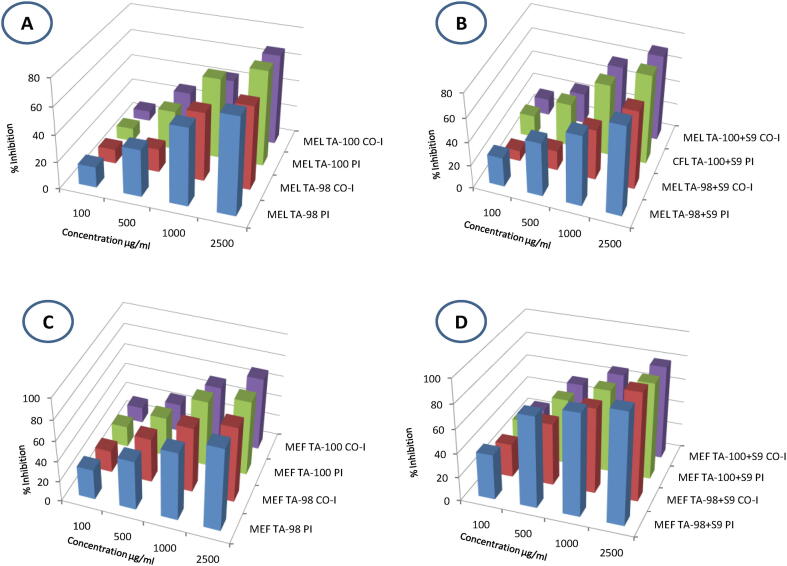
The antimutagenic activity of MEL in TA-98 strain against NPD mutagen, in terms of % inhibition, was 70.53% in pre-incubation (PI) mode and 61.09% in the co-incubation (Co-I) mode, whereas, against sodium azide mutagen in the TA-100 strain, the inhibition was 70.49% in PI and 67.16% in Co-I mode at the maximum concentration of 2500 µg/0.1 ml [Fig. 2 (A)]. On the other hand, against 2-AF (S9 dependent mutagen) highest tested concentration of MEL decreased the number of histidine revertants by 75.94% and 66.33% in TA-98 PI and Co-I mode respectively, whereas, in TA-100 strain 74.80% and 71.54% inhibition was noted in PI and Co-I mode correspondingly at the same concentration [Fig. 2 (B)].
Fig. 2.
Antimutagenic activity (%) of Rhododendron arboreum extracts in TA-98 and TA-100 strains of Salmonella typhimurium. Pre-incubation (PI) and co-incubation (Co-I) modes of experimentation; without metabolic activation against direct acting mutagens, 4-nitro-O-phenylenediamine for TA-98 & sodium azide for TA-100 and with metabolic activation against 2-aminofluorene using rat liver homogenate (S9). (A) methanol extract of leaves (MEL) without metabolic activation; (B) methanol extract of leaves (MEL) with metabolic activation; (C) methanol extract of flowers (MEF) without metabolic activation; (D) methanol extract of flowers (MEF) with metabolic activation.
Antimutagenic action of methanol extract of R. arboreum flowers is represented in Table 3. In TA-98 strain, against the mutagen NPD, 80.86% and 73.44% activity was revealed by MEF in both PI and Co-I mode correspondingly at maximum concentration applied (2500 µg/0.1 ml). Against sodium azide in TA-100 tester strain, MEF showed an inhibitory activity of 72.34% and 70.21% in PI and Co-I mode of experiments respectively at same concentration [Fig. 2 (C)]. Against S9 mediated mutagenicity of 2-AF, the MEF successfully decreased the number of TA-98 revertant colonies at concentration of 2500 µg/0.1 ml in PI and Co-I with inhibition of 91.85% and 89.78% respectively. In TA-100 strain PI and Co-I mode of treatment of MEF detained the mutagenicity of 2-AF up to 80.87% and 78.86% respectively at same concentration [Fig. 2(D)].
3.6. Cancer cell growth inhibition activity
Amongst all the three cell lines, (HeLa, MCF7 and A549), the methanol extract of R. arboreum leaves showed highest cytotoxic effect against A549 cell line with % inhibition of 17.73 ± 0.773% at minimum tested concentration (30 µg/ml) to 75.08 ± 1.68% at maximum tested concentration (500 µg/ml), followed by MCF7 (15.45 ± 0.54% at 30 µg/ml to 73.57 ± 1.27% at 500 µg/ml) and Hela (17.04 ± 0.57% at 30 µg/ml to 64.62 ± 2.65% at 500 µg/ml) cell lines (Table 1).
The methanol extract of R. arboreum flowers showed maximum cytotoxic activity against MCF7 cell line with % inhibition of 19.04 ± 1.43% at minimum tested concentration (30 µg/ml) to 84.93 ± 1.17% at maximum tested concentration (500 µg/ml), followed by HeLa (8.29 ± 1.76% at 30 µg/ml to 53.11 ± 2.84% at 500 µg/ml) and A549 (13.05 ± 1.58% at 30 µg/ml to 45.92 ± 2.43% at 500 µg/ml) cell lines (Table 1).
4. Discussion
The UHPLC analysis revealed that ellagic acid was most abundant polyphenol in both the extracts following the trend as follows: MEF (148.48 ppm) > MEL (98.003 ppm). The perusal of literature revealed that polyphenols are the widespread and prevalent class of phytochemicals. These protect the plants from a variety of damage as their dynamic redox metal ions chelate the free radicals or inactivate the ROS (Link et al., 2010). The intake of polyphenols in human diet is through plant products like fruits and vegetables (de Andrade et al., 2014). El-Soud et al. (2013) reported the osmotic stress alleviating potential of ellagic acid on chickpea. It is also reported to protect the human keratinocyte cells in opposition to UVA-stimulated damage by the regulating the antioxidant genes (Hseu et al., 2012). Ellagic acid also prevents colon cancer in animals (Whitley et al., 2005). In vitro anti-proliferative activities of ellagic acid were also confirmed by Losso et al. (2004). Gallic acid exhibits anticancer activity against HCT15 and MDA MB 231 cancer cell lines (Devi et al., 2014).
To find out the other phyto-constituents of the R. arboreum leaves and flowers, the extracts were analyzed using the GC-MS technique. It was observed that linoleic acid, flavone 4′-OH, Benzenepropanoic acid, hexadecanoic acid (CAS) palmitic acid and 9,12-Octadecadienoic acid were commonly present in leaves as well as flowers. The compounds identified in the present study belong to different categories e.g. palmitic acid and linoleic acid are fatty acids; Palmitic acid has excellent potential as free scavenger of free radicals (Kim et al., 2006). 9,12-Octadecadien-1-ol possess healing potential against arthritis and inflammation (Kim et al., 2006). 9,12 octadecadienoic acid can be used as anti-inflammatory, anti-microbial and anti-arthritis agent (Hornby et al., 2001). Linoleic acid, have antimicrobial activities (Udgire and Pathade, 2013). The existence of these phyto-constituents in R. arboreum points towards its expectant candidature for remedial use.
Amino acids are linked with the biosynthesis of vitamins, proteins and various enzymes that help in combating the environmental stress (Anjum et al., 2012). In this study, the extracts of R. arboreum leaves and flowers were tested qualitatively as well as quantitatively for amino acids namely aspartic acid, glutamic acid, serine, glutamine, citrulline, threonine, alanine, b-alanine, tyrosine, cystine, tryphtophan, isoleucine, leucine, lysine and proline using amino acid analyzer. MEL showed the presence of 11 amino acids, whereas, 10 amino acids were identified in MEF. These amino acids help in stress resistance (Zagorchev et al., 2013). The aspartic acid possesses antimicrobial activity and is also used as dietary supplement for sportspersons (Aiyelabola et al., 2016). Aspartic acid and glycine possess antimicrobial and cytotoxic activities (Aiyelabola et al., 2017). Glutamic acid and glutamine are reported to possess antibacterial properties against Pseudomonas aeruginosa and Bacillus cereus (Abdel-Rahman et al., 2017). Derivatives of citrulline and arginine are antitumour agents (Rad-Niknam and Sharples, 2010).
The results recognized that the radical extinguishing property of the both extracts were dosage reliant. In methanol extract of leaves antioxidant activity was in the order: lipid peroxidation inhibitory assay (IC50 103.6 µg/ml) > nitric oxide scavenging assay (IC50 179.52 µg/ml). In MEF better activity was observed in nitric oxide scavenging assay (IC50150.13 µg/ml) than lipid peroxidation inhibitory assay (IC50271.17 µg/ml). The possible reason for the antioxidant potential of R. arboreum could be the fact that the phyto-constituents of the extracts either efficiently scavenged or inhibited the production of the free radicals. It has been reported that the antioxidant properties of plants are due to their bioactive phytochemicals like ellagic acid, rutin, gallic acid and polyphenols (Proestos et al., 2005, Gupta et al., 2011, Asgar, 2013). There are several other reports of antioxidant activity of plant extracts and their relation to the phytochemicals. The antioxidant activity of Curcuma caesia Roxb. rhizome extracts were reported by Devi et al. (2015). The antioxidant activity of Emex spinosus (L.), Asphodelus tenuifolius Cav., and Aizoon canariense L. was evaluated by Al-Laith et al. (2015) using different assays and strong association was observed among the antioxidant activity and free phenolics. Likewise, Vijayakumar et al. (2013) reported the in vitro antioxidant activity of Illicium griffithi seeds. There are some other studies reporting the antioxidant activity of Plukenetia volubilis Linneo, Theobroma cacao, Artemisia nilagirica L., Zuccagnia punctata Cav. (Zp), Citrus sinensis, Citrus latifolia, Tragopogon longirostis and its correlation with the polyphenols, amino acids and other phyto-constituents. (Nascimento et al., 2013, Baharum et al.,2014, Sarac, 2015, Carabajal et al., 2017, Toscano-Garibay et al., 2017).
The results indicated that the leaf and flowers extracts hold the capability to defend the DNA of Salmonella typhimurium from frame movement or base pair transmutations. More prominent antimutagenic activity was observed against S9 dependent mutagen than direct acting mutagens. Against NDP as well as S9 mediated mutagenicity of 2-AF in both TA-98 and TA-100 strains, the MEF was noted to be better antimutagen than MEL as it successfully decreased the number of revertant colonies in both PI and Co-I modes of treatment at highest tested concentration. There are several reports which correlate the antimutagenic activity of plants to the presence of phytochemicals like polyphenols which are capable of scavenging the free radicals or stimulate the antioxidant enzymes (Hochstein and Atallah, 1988, Horn and Vargas, 2003, Aqil et al., 2008). Curcuma caesia Roxb. rhizome extracts possess antimutagenic activity in S. typhimurium (Devi et al., 2015). The antimutagenic activity of Cinnamomum zeylanicum fruit extracts against TA-100 strain of S. typhimurium was observed and phenolics in fruits were reported to be accountable in favour of the antimutagenic activity (Jayaprakasha et al., 2007). Maytenus ilicifolia was reported to be antimutagenic and rich in compounds of the flavonoid and tannin groups (Horn and Vargas, 2003). Masfria and Dalimunthe (2017) reported the antimutagenic activity of Rhaphidophora pinnata (L.f) Schott Leaves. Zuccagnia punctata Cav. (Zp), Citrus sinensis, Citrus latifolia, Pfaffia glomerata, Tragopogon longirostis, Glycosmis pentaphylla and Tabernaemontana coronaria were also reported to possess antimutagenic activity (Sarac, 2015, Almeida et al., 2017, Carabajal et al., 2017, Toscano-Garibay et al., 2017, Kumar et al., 2018). It was reported that antimutagenic activity have direct relationship with phenolic content (Makhafola et al., 2016).
The capability of extracts of R. arboreum leaves and flowers to restrain the in vitro growth of cancer cell lines (HeLa, MCF7 and A549) was measured by MTT assay. Both the extracts showed dose dependent cytotoxic activity. Polyphenols are reported to be anticancer agents (Dai and Mumper, 2010, Asgar, 2013, Benayad et al., 2014), therefore, the cancer cell growth inhibition activity of extracts in present study can be attributed to the polyphenols identified with UHPLC analysis especially the ellagic acid. There are several reports of antiproliferative activities of other plant extracts for example, Ravi et al. (2012) reported the dose dependent cytotoxicity of Pupalia lappacea against human embryonic kidney cells. In a different study, Kumar et al. (2012) reported the antiproliferative activity of Helicteres isora fruit extracts against human lung cancer cells. Gandhiappan and Rengasamy (2012) reported the antiproliferative activity of Solanum anguivi leaves against HEpG-2 and MCF-7 cancer cell lines. The antiproliferative activity of Arbutus andrachne L., and Teucrium polium L. was reported against human tumor cell lines (Abu-rish et al., 2016). In vitro antiproliferative activity of Theobroma cacao, Origanum compactum Benth., Arbutus unedo L. and Triumfetta welwitschii is reported (Baharum et al.,2014, Karakas et al., 2015, Moyo and Mukanganyama, 2015, Yaglıoglu et al., 2015, Bouyahya et al., 2018).
5. Conclusion
The study discloses the significant antioxidant, antimutagenic and cancer cells growth inhibition activities of R. arboreum. We recommend that those activities may be the effect of the collective consequence of the phytochemicals recognized by UHPLC, GC-MS and amino acid analyzer. Therefore, taking above findings into consideration, the R. arboreum extracts may have future prospect as primary agent in pharmaceutical drug production to treat the various oxidative stress related diseases including DNA mutations and cancer.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2019/116), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Renu Bhardwaj, Email: renubhardwaj82@gmail.com.
Parvaiz Ahmad, Email: parvaizbot@yahoo.com.
References
- Abdel-Rahman L.H., Abu-Dief A.M., Ismail N.M., Ismael M. Synthesis, characterization, and biological activity of new mixed ligand transition metal complexes of glutamine, glutaric, and glutamic acid with nitrogen based ligands. Inorg. Nano-Met. Chem. 2017;47:467–480. doi: 10.1080/15533174.2015.1137057. [DOI] [Google Scholar]
- Abu-rish E.Y., Kasabri V., Hudaib M.M., Mashalla S.H., AlAlawi L.H., Tawaha K., Mohammad M.K., Mohamed Y.S., Bustanji Y. Evaluation of antiproliferative activity of some traditional anticancer herbal remedies from Jordan. Tropical J. Pharm. Res. 2016;15:469. doi: 10.4314/tjpr.v15i3.6. [DOI] [Google Scholar]
- Aiyelabola T., Akinkunmi E., Ojo I., Obuotor E., Adebajo C., Isabirye D. Syntheses, characterization, resolution, and biological studies of coordination compounds of aspartic acid and glycine. Bioinorg. Chem. Appl. 2017;2017:1–15. doi: 10.1155/2017/2956145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiyelabola T.O., Isabirye D.A., Akinkunmi E.O., Ogunkunle O.A., Ojo I.A.O. Synthesis, characterization, and antimicrobial activities of coordination compounds of aspartic acid. J. Chem. 2016;2016:1–8. doi: 10.1155/2016/7317015. [DOI] [Google Scholar]
- Al-Laith A.A., Alkhuzai J., Freije A. Assessment of antioxidant activities of three wild medicinal plants from Bahrain. Arab. J. Chem. 2015 doi: 10.1016/j.arabjc.2015.03.004. [DOI] [Google Scholar]
- Almeida I.V., Düsman E., Mattge G.I., Toledo F., Reusing A.F., Vicentini V.E.P. In vivo antimutagenic activity of the medicinal plants Pfaffia glomerata (Brazilian ginseng) and Ginkgo biloba. Gen. Mol. Res. 2017;16 doi: 10.4238/gmr16039785. [DOI] [PubMed] [Google Scholar]
- Anjum S.A., Farooq M., Xie X.-y., Liu X.-j., Ijaz M.F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hort. 2012;140:66–73. doi: 10.1016/j.scienta.2012.03.028. [DOI] [Google Scholar]
- Aqil, F., Zahin, M., Ahmad, I., 2008. Antimutagenic activity of methanolic extracts of four ayurvedic medicinal plants. [PubMed]
- Asgar A. Anti-diabetic potential of phenolic compounds: a review. Int. J. Food Prop. 2013;16:91–103. doi: 10.1080/10942912.2011.595864. [DOI] [Google Scholar]
- Baharum Z., Akim A., Taufiq-Yap Y., Hamid R., Kasran R. In vitro antioxidant and antiproliferative activities of methanolic plant part extracts of theobroma cacao. Molecules. 2014;19:18317–18331. doi: 10.3390/molecules191118317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala S., Grover I.S. Antimutagenicity of some citrus fruits in Salmonella typhimurium. Mutation Res./Genetic Toxicol. 1989;222:141–148. doi: 10.1016/0165-1218(89)90129-8. Available from. [DOI] [PubMed] [Google Scholar]
- Benayad Z., Martinez-Villaluenga C., Frias J., Gomez-Cordoves C., Es-Safi N.E. Phenolic composition, antioxidant and anti-inflammatory activities of extracts from Moroccan Opuntia ficus-indica flowers obtained by different extraction methods. Ind. Crops Prod. 2014;62:412–420. doi: 10.1016/j.indcrop.2014.08.046. [DOI] [Google Scholar]
- Bennick A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Medicine. 2002;13:184–196. doi: 10.1177/154411130201300208. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S.K. Pointer Publishers; Jaipur: 1998. Handbook of Medicinal Plants. [Google Scholar]
- Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/wox.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyahya A., Bakri Y., Et-Touys A., Assemian I.C.C., Abrini J., Dakka N. In vitro antiproliferative activity of selected medicinal plants from the North-West of Morocco on several cancer cell lines. Eur. J. Integr. Med. 2018;18:23–29. doi: 10.1016/j.eujim.2018.01.001. [DOI] [Google Scholar]
- Carabajal M.P.A., Isla M.I., Zampini I.C. Evaluation of antioxidant and antimutagenic activity of herbal teas from native plants used in traditional medicine in Argentina. S. Afr. J. Bot. 2017;110:258–265. doi: 10.1016/j.sajb.2016.10.006. [DOI] [Google Scholar]
- Dai J., Mumper R.J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade E.F., Leone R.D.S., Ellendersen L.N., Masson M.L. Phenolic profile and antioxidant activity of extracts of leaves and flowers of yacon (Smallanthus sonchifolius) Indust. Crops Prod. 2014;62:499–506. doi: 10.1016/j.indcrop.2014.09.025. [DOI] [Google Scholar]
- Devi H.P., Mazumder P.B., Devi L.P. Antioxidant and antimutagenic activity of Curcuma caesia Roxb. rhizome extracts. Toxicol. Rep. 2015;2:423–428. doi: 10.1016/j.toxrep.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi Y.P., Uma A., Narasu M.L., Kalyani C. Anticancer activity of gallic acid on cancer cell lines, HCT-15 and MDA MB 231. Int. J. Res. Appl. Nat. Soc. Sci. 2014;2:269–272. [Google Scholar]
- El-Soud W.A., Hegab M.M., AbdElgawad H., Zinta G., Asard H. Ability of ellagic acid to alleviate osmotic stress on chickpea seedlings. Plant Physiol. Biochem. 2013;71:173–183. doi: 10.1016/j.plaphy.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Gandhiappan J., Rengasamy R. Antiproliferative activity of Solanum anguivi against cancer cell lines. Der Pharm. Lett. 2012;4:875–880. [Google Scholar]
- Garner R.C., Miller E.C., Miller J.A. Liver microsomal metabolism of aflatoxin B1 to a reactive derivative toxic to Salmonella typhimurium TA 1530. Cancer Res. 1972;32:2058–2066. [PubMed] [Google Scholar]
- Gautam V., Kohli S., Arora S., Bhardwaj R., Kazi M., Ahmad A., Raish M., Ganaie M., Ahmad P. Antioxidant and antimutagenic activities of different fractions from the leaves of Rhododendron arboreum Sm. and their GC-MS profiling. Molecules. 2018;23:2239. doi: 10.3390/molecules23092239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Sharma A.K., Sharma M.C., Dobhal M.P., Gupta R.S. Evaluation of antidiabetic and antioxidant potential of lupeol in experimental hyperglycaemia. Nat. Prod. Res. 2011;26:1125–1129. doi: 10.1080/14786419.2011.560845. [DOI] [PubMed] [Google Scholar]
- Hagerman A.E., Riedl K.M., Jones G.A., Sovik K.N., Ritchard N.T., Hartzfeld P.W., Riechel T.L. High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J. Agric. Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J.M. Oxford University Press; USA: 2015. Free Radicals in Biology and Medicine. [Google Scholar]
- Hela A., Abdullah A. Antioxidant and antimicrobial activities of methanol extracts of some Verbena species: In vitro evaluation of antioxidant and antimicrobial activity in relation to polyphenolic content. J. Appl. Sci. Res. 2010;6:683–689. [Google Scholar]
- Hochstein P., Atallah A.S. The nature of oxidants and antioxidant systems in the inhibition of mutation and cancer. Mutation Res./Fundamental Mol. Mech. Mutagenesis. 1988;202:363–375. doi: 10.1016/0027-5107(88)90198-4. [DOI] [PubMed] [Google Scholar]
- Horn R.C., Vargas V.M.F. Antimutagenic activity of extracts of natural substances in the Salmonella/microsome assay. Mutagenesis. 2003;18:113–118. doi: 10.1093/mutage/18.2.113. [DOI] [PubMed] [Google Scholar]
- Hornby J.M., Jensen E.C., Lisec A.D., Tasto J.J., Jahnke B., Shoemaker R., Dussault P., Nickerson K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by Farnesol. Appl. Environ. Microbiol. 2001;67:2982–2992. doi: 10.1128/aem.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hseu Y.-C., Chou C.-W., Senthil Kumar K.J., Fu K.-T., Wang H.-M., Hsu L.-S., Kuo Y.-H., Wu C.-R., Chen S.-C., Yang H.-L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem. Toxicol. 2012;50:1245–1255. doi: 10.1016/j.fct.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha G.K., Negi P.S., Jena B.S., Jagan Mohan Rao L. Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts. J. Food Composition Anal. 2007;20:330–336. doi: 10.1016/j.jfca.2006.07.006. [DOI] [Google Scholar]
- Karakas F.P., Yidirim A.B., Bayram R., Yavuz M.Z., Gepdiremen A., Turker A.U. Antiproliferative activity of some medicinal plants on human breast and hepatocellular carcinoma cell lines and their phenolic contents. Trop. J. Pharm. Res. 2015;14:1787. doi: 10.4314/tjpr.v14i10.8. [DOI] [Google Scholar]
- Kemertelidze E.P., Shalashvili K.G., Korsantiya B.M., Nizharadze N.O., Chipashvili N.S. Therapeutic effect of phenolic compounds isolated from Rhododendron ungernii leaves. Pharm. Chem. J. 2007;41:10–13. [Google Scholar]
- Kim S., Jeong S., Park W., Nam K., Ahn D., Lee S. Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts. Food Chem. 2006;97:472–479. doi: 10.1016/j.foodchem.2005.05.027. [DOI] [Google Scholar]
- Kiruba S., Mahesh M., Nisha S.R., Paul Z.M., Jeeva S. Phytochemical analysis of the flower extracts of Rhododendron arboreum Sm. ssp. nilagiricum (Zenker) Tagg. Asian Pac. J. Tropical Biomed. 2011:S284–S286. [Google Scholar]
- Kumar A., Banerjee N., Singamaneni V.K., Dokuparthi S., Chakrabarti T., Mukhopadhyay S. Phytochemical investigations and evaluation of antimutagenic activity of the alcoholic extract of Glycosmis pentaphylla and Tabernaemontana coronaria by Ames test. Nat. Prod. Res. 2018;32:582–587. doi: 10.1080/14786419.2017.1318384. [DOI] [PubMed] [Google Scholar]
- Kumar T.M., Christy A.M.V., Ramya R.C.S., Malaisamy M., Sivaraj C., Arjun P., Raaman N., Balasubramanian K. Antioxidant and anticancer activity of Helicteres isora dried fruit solvent extracts. J. Acad. Indus. Res. 2012;1:148–152. [Google Scholar]
- Laloo R.C., Kharlukhi L., Jeeva S., Mishra B.P. Status of medicinal plants in the disturbed and the undisturbed sacred forests of Meghalaya, northeast India: population structure and regeneration efficacy of some important species. Curr. Sci. 2006;90:225–232. [Google Scholar]
- Link A., Balaguer F., Goel A. Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem. Pharmacol. 2010;80:1771–1792. doi: 10.1016/j.bcp.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losso J., Bansode R., Trappeyii A., Bawadi H., Truax R. In vitro anti-proliferative activities of ellagic acid. J. Nutr. Biochem. 2004;15:672–678. doi: 10.1016/j.jnutbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Makhafola T.J., Elgorashi E.E., McGaw L.J., Verschaeve L., Eloff J.N. The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant species with high antioxidant activity. BMC Complement. Alternat. Med. 2016;16 doi: 10.1186/s12906-016-1437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcocci L., Maguire J.J., Droylefaix M.T., Packer L. The nitric oxide-scavenging properties of Ginkgo biloba Extract EGb 761. Biochem. Biophys. Res. Commun. 1994;201:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- Markesbery W.R. Oxidative stress hypothesis in Alzheimer's disease. Free Radical Biol. Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutation Res./Environ. Mutagenesis Related Subjects. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Masfria S., Dalimunthe A. Antimutagenic Activity of Ethanol Extract of Rhaphidophora pinnata (L.f) Schott Leaves on Mice. Scientia Pharmaceutica. 2017;85:7. doi: 10.3390/scipharm85010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Khan M.A., Ashraf M.Q., Rizwana A. Traditional use of herbs, shrubs and trees of Shogran valley, Mansehra, Pakistan. Pak. J. Biol. Sci. 2001;4:1101–1107. [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Moyo B., Mukanganyama S. Antiproliferative Activity of T. welwitschii Extract on Jurkat T CellsIn Vitro. BioMed. Res. Int. 2015;2015:1–10. doi: 10.1155/2015/817624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento A.K.L., Melo-Silveira R.F., Dantas-Santos N., Fernandes J.M., Zucolotto S.M., Rocha H.A.O., Scortecci K.C. Antioxidant and antiproliferative activities of leaf extracts from Plukenetia volubilis Linneo (Euphorbiaceae) Evidence-Based Complement. Alternat. Med. 2013;2013:1–10. doi: 10.1155/2013/950272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painuli S., Rai N., Kumar N. Gas chromatography and mass spectrometry analysis of methanolic extract of leaves of Rhododendron arboreum. Asian J. Pharm. Clin. Res. 2016;9(1):66–69. [Google Scholar]
- Proestos C., Chorianopoulos N., Nychas G.J.E., Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005;53:1190–1195. doi: 10.1021/jf040083t. [DOI] [PubMed] [Google Scholar]
- Racchi M. Antioxidant defenses in plants with attention to prunus and citrus spp. Antioxidants. 2013;2:340–369. doi: 10.3390/antiox2040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad-Niknam M., Sharples D. Potential antitumor agents. Synthesis, DNA binding studies, and biological activity of 9-aminoacridinyl derivatives of citrulline and arginine. ChemInform. 2010;22 doi: 10.1002/chin.199101221. [DOI] [PubMed] [Google Scholar]
- Ravi A., Alvala M., Sama V., Kalle A.M., Irlapati V.K., Reddy B.M. Anticancer activity of Pupalia lappacea on chronic myeloid leukemia K562 cells. DARU J. Pharm. Sci. 2012;20 doi: 10.1186/2008-2231-20-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy J.D., Handique A.K., Barua C.C., Talukdar A., Ahmed F.A., Barua I.C. Evaluation of phytoconstituents and assessment of adaptogenic activity in vivo in various extracts of Rhododendron arboreum(leaves) J. Pharm. Biol. Res. 2014;2(2):49–56. [Google Scholar]
- Sarac, N., 2015. Antioxidant, mutagenic, and antimutagenic activities of Tragopogon longirostis var. longirostis, an edible wild plant in Turkey. Indian J. Pharmacol. 47, 414. 10.4103/0253-7613.161267. [DOI] [PMC free article] [PubMed]
- Shyam S.A., Kalpana S. Anti-inflammatory activity of flowers of Rhododendron arboreum (SMITH) in rat’s hind paw edema induced by various phlogistic agents. Indian J. Pharmacol. 1988;20:86–89. [Google Scholar]
- Smith M.A., Perry G., Richey P.L., Sayrec L.M., Anderson V.E., Beal M.F., Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y.J., Forman H.J., Sevanian A. Oxidants as stimulators of signal transduction. Free Radical Biol. Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- Toscano-Garibay J.D., Arriaga-Alba M., Sánchez-Navarrete J., Mendoza-García M., Flores-Estrada J.J., Moreno-Eutimio M.A., Espinosa-Aguirre J.J., González-Ávila M., Ruiz-Pérez N.J. Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-11818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udgire M., Pathade G. Evaluation of antimicrobial activities and phytochemical constituents of extracts of Valeriana wallichii. Asian J. Plant Sci. Res. 2013;3:55–59. [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vijayakumar A., Duraipandiyan V., Jeyaraj B., Agastian P., Raj M.K., Ignacimuthu S. Phytochemical analysis and in vitro antimicrobial activity of Illicium griffithii Hook. f. & Thoms extracts. Asian Pac. J. Tropical Disease. 2013;2:190–199. doi: 10.1016/s2222-1808(12)60045-0. [DOI] [Google Scholar]
- Watt G.A. Dictionary of the economic natural products of India. Harvard University Supt of Govt Prtg. 1982:492–495. [Google Scholar]
- Weinbrenner T., Cladellas M., Isabel Covas M., Fitó M., Tomás M., Sentí M., Bruguera J., Marrugat J. High oxidative stress in patients with stable coronary heart disease. Atherosclerosis. 2003;168:99–106. doi: 10.1016/s0021-9150(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Whitley A.C., Sweet D.H., Walle T. The dietary polyphenol ellagic acid is a potent inhibitor of hOAT1. Drug Metab. Disposition. 2005;33:1097–1100. doi: 10.1124/dmd.105.004275. [DOI] [PubMed] [Google Scholar]
- Yaglıoglu S.A., Eser F., Tekin S., Onal A. Antiproliferative activities of several plant extracts from Turkey on rat brain tumor and human cervix carcinoma cell lines. Front. Life Sci. 2015;9:69–74. doi: 10.1080/21553769.2015.1089949. [DOI] [Google Scholar]
- Zagorchev L., Seal C., Kranner I., Odjakova M. A central role for thiols in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2013;14:7405–7432. doi: 10.3390/ijms14047405. [DOI] [PMC free article] [PubMed] [Google Scholar]