Abstract
Microbial enhanced oil recovery (MEOR) is a kind of enhanced oil recovery (EOR) development, often used as a tertiary stage where oil recovery is no longer possible utilizing primary and secondary conventional techniques. Among a few potential natural operators valuable for MEOR, biosurfactants, biopolymers and biosurfactant based nanoparticles assume key jobs. Biosurfactant which are produced by microorganisms’ act as are surface active agents that can be used as an alternative to chemically synthesized surfactants. Pseudomonas aeruginosa TEN01, a gram-negative bacterium isolated from the petroleum industry is a potential biosurfactant (Rhamnolipid) producer using cassava waste as the substrate. This work focuses on production and characterization of rhamnolipid from P. aeruginosa TEN01 and its use in enhanced oil recovery. The effectiveness of Chitosan that is deacetylated form of chitin which is a biopolymer that provides density and viscosity to the fluids is not known in enhanced oil recovery yet and so it is studied. Moreover, the fabrication of biosurfactant-mediated silver nanocrystals and its application in enhanced oil recovery is also studied. Sand-Pack column was constructed and the mechanism of oil recovery in the column was studied. While incubating the crude oil containing sand packed column with Biosurfactant-biopolymer and brine flooding in the ratio of 1:2, and Biosurfactant incubation - flooding with 3 g/l of biopolymer was found to be 34.28% and 44.5% respectively. The biosurfactant based silver nanoparticles are non-toxic and have better stability when compared to chemically synthesized silver nanoparticles. The oil recovery percentage by chemical based Ag NPs and biosurfactant based Ag NPs are 14.94% and 14.28% respectively.
Keywords: Nanotechnology, Pseudomonas aeruginosa TEN01, Interfacial tension, Sand packed column, Enhanced oil recovery
1. Introduction
From the earliest starting point of civic establishments, we are constantly subject to energizes, for example, wood, whale-oil or petroleum derivatives - coal, and raw petroleum related items (lamp oil, diesel, oil). Among each one of those diverse sort of energizes, non-renewable energy source assumed a major job in mechanical goals (Silva et al., 2014). Increase in oil costs based on market interest disparities are observed as of late resulted a decrease which is relied upon to remain at 30–60 $/bbl oil for a prolonged duration (Schulz, 2016, Banat, 1995, McInerney et al., 2005). Current situation of such an uncertain market at oil costs is a very tough path ahead for oil ventures and those nations which are exceptionally reliant on oil-based economy. Nevertheless, the oil industry being utilized to face such situation and such frantic occasions consistently brought space for enhancements and overhauling of innovations to guarantee progressively prudent and compelling results. These developments included enhanced recovery of oil created to monetarily increase the extraction of oil and recovery yields (Jha, 1999, Stosur, 2003, Elraies and Tan, 2012, Al-Wahaibi et al., 2016, Bachmann et al., 2014, Al-Sulaimani et al., 2011, Rudyk et al., 2017, Simjoo et al., 2019).
Surfactants are amphiphilic agents finds its application in various areas (Mulligan and Gibbs, 1993, Amani et al., 2010, Amani et al., 2013). The petroleum industry has been significant user of surfactants because the solubility of petroleum components is increased (Falatko, 1991). Although chemical surfactants are desired agents for EOR operations, but, there are some problems in environment friendly (Levitt et al., 2006, Al-Sulaimani et al., 2011, Gogoi, 2011, Mohsenzadeh et al., 2015). Naturally produced biosurfactants are an alternative rather than chemical surfactants. Low toxicity, good biodegradability, low critical micelle concentration (CMC) of biosurfactant make its more advantageous than chemical surfactants (Desai and Banat, 1997, Rahman and Gakpe, 2008, Zhao et al., 2015, Joshi and Abed, 2017).
Rhamnolipids are glycolipids generally isolated from Pseudomonas aeruginosa. They show moderately greater surface activities and are produced in enormous sum in short incubation periods (Jarvis and Johnson, 1949, Abdel et al., 2010). A rhamnolipids surfactant is one of the compounds which has the ability to release the oil more effectively and can remove the hydrocarbon mixture from the soil. As the rhamnolipid biosurfactant cannot be easily absorbed by the soil it is regarded as the best alternative to chemical surfactant in the field of enhanced oil recovery (Pornsunthorntawee et al., 2008, Reddy et al., 2009). Because of their quantum size and surface impacts, metal nanoparticles have potential application in different fields that bring in novel physical, substance, attractive and auxiliary properties that mass or individual atoms don't show. The advancement of ecologically benign technique for the nanoparticles synthesis is emerging as a significant part of nanotechnology (Li et al., 2013). For orchestrating naturally benign nanoparticles we use biosurfactants got from P. aeruginosa TEN01, which have properties like biodegradable, less dangerous and it doesn't contaminate the earth and furthermore it assumes a key job in the decrease of the metal antecedent, just as in the stabilization of the subsequent nanoparticles.
As of late metal nanoparticles helped overwhelming recovery of oil has been accounted for with efficient recovery proficiency at generally low financial and ecological expenses (Loria et al., 2011, Guo et al., 2015). These nanometer measured particles empower the adequate contact between metallic catalyst and oil molecules and quickens the splitting and hydrogenation of hydrocarbons. Profiting by the benefits of nanomaterials, for example, high inalienable catalytic activity, high surface area to volume ratio, effortless transport inside permeable rocks and controllable combination for explicit capacities, this inventive methodology for overwhelming oil generation prompts huge improvement for substantial oil overhauling and recovery (Nitschke and Maria, 2006). The aim of this work is to produce biosurfactant, biosurfactant based silver nanoparticle and to prepare biopolymer solution and use these bioproducts in enhanced oil recovery.
2. Material and methods
2.1. Production of biosurfactant using cassava waste
Biosurfactant production was carried out using production medium consisting of 9% (w/v) of cassava waste, 1.2% (w/v) of glycerol, 0.3% (w/v) sodium nitrate, and 1% P. aeruginosa TEN01 was inoculated in the production medium. Then it was incubated on 37 °C for 7 days with shaking conditions (Kaskatepe et al., 2015).
2.2. Biopolymer solution
Chitosan is dissolved in 2% acetic acid and constantly stirred at 60 °C until it dissolves completely.
2.3. Construction of a sand pack column
A glass column (vertically oriented 35 × 2 cm dimensions) was packed with sterilized sand. To maintain a uniform packing, the sand was poured into the column in small amounts with gentle tapping (Suthar et al., 2008, Li et al., 2002).
2.4. Porosity of the soil sample
Pore space is the amount of empty space in the rock or earth’s surface. Porosity is the measurement of how much water a soil can hold. In other words, porosity is the percentage of material’s total volume. Measure 100 ml of sample in the cylinder and pour the water in it slowly until the water reaches the top layer of the soil. The amount of water used to fill the sand is pore space and % porosity is calculated using pore space and total volume used.
2.5. Permeability of the soil sample
The term permeability denotes efficiency of water flow through the earth surface. Permeability is measured in terms of time the water takes to reach the bottom of the sample.
2.6. Operation of sand pack column
100 g of sterile sand was packed in the column. Volume of 5% brine solution was added to saturate the column. This is the process called brine saturation. The volume of brine saturating the column represents the pore volume. Then the oil is added. Now oil stats to replace the brine solution. The overall amount of oil packed throughout the column represents the original oil in place (OOIP). By this process, brine gets discharged and certain amount of oil gets eluted. The amount of oil eluted represents the Initial oil saturation (Soi). Certain amount of oil remains in the column apart from getting eluted. This is the residual oil saturation (Sor) (Suthar et al., 2008). The various process of oil recovery using biosurfactant, biopolymer, nanoparticle incubation is carried out with the residual oil.
The oil recovered after the incubation process represents the final oil recovery (Gao and Zekri, 2011).
2.6.1. Combined effect of biosurfactant and biopolymer (Chitosan) in Ex-situ EOR
After completion of previously mentioned processes, biosurfactant and biopolymer was added in varying ratios in the sand pack column and incubated for 24 h (Shah, 2012). Again, the column was flooded with brine solution to recover the released oil.
2.6.2. Biopolymer (Chitosan) flooding in Ex-situ EOR
Here, rather than brine flooding, biopolymer was flooded soon after Biosurfactant incubation. (i.e.) Biosurfactant was passed through the column and incubated for 24 h after which biopolymer was flooded to recover the oil from the column (Gao, 2016).
2.6.3. Percentage of oil recovery
The percentage of oil recovered was calculated by using the parameters OOIP and Sor (Geetha et al., 2018).
2.7. Biofabrication of AgNPs
Here, the nanoparticles were synthesized by using chemical surfactant and biosurfactant. Firstly by using SDS, 20 ml of 33.7 mg of aqueous AgNO3 solution was prepared and mixed with 20 ml of SDS under vigorous stirring for 10 min. Then 20 ml of 70 mg aqueous NaBH4 was prepared and added to above mixture and then the solution stirred until the reddish brown color appears. After color changes, λmax is measured using UV–Vis spectrometer from wavelength 200–800 nm. Secondly by biosurfactant, 20 ml of 33.7 mg of aqueous AgNO3 solution was prepared and mixed with 10 ml of supernatant from production medium under vigorous stirring for 10 min. Then 20 ml of 70 mg aqueous NaBH4 was prepared and added to above mixture and then the solution stirred until the reddish brown color appears. After color changes, λmax is measured using UV–Vis spectrometer (Sivasubramani and Selvaraj, 2017).
2.8. Characterization of Ag NPs
The structural properties of the samples was checked by and Wide angle X Ray Diffraction PAN analytical using Cu Kα radiation (λ = 0.15406 nm), operating at 20 mA and 40 KV at the scan rate 5° per min over the length of (2θ = 30–80°) and using FESEM performed the morphology-Visible spectroscopy were measured by Analytek Jenaat the wavelength range of 400–800 nm optical absorbance analysis. The size and stability of the synthesized silver nanoparticles by both chemical surfactant and biosurfactant were characterized by using Malvern zetasizer. In Malvern zetasizer, dynamic light scattering (DLS) technique is used for determining the size and stability of particle.
2.9. Sand pack column for AgNPs
The same procedure for operation of sand pack column was followed for biosurfactant based silver nanoparticle and thus percentage of oil recovery was calculated.
3. Results and discussion
3.1. Extraction of biosurfactant
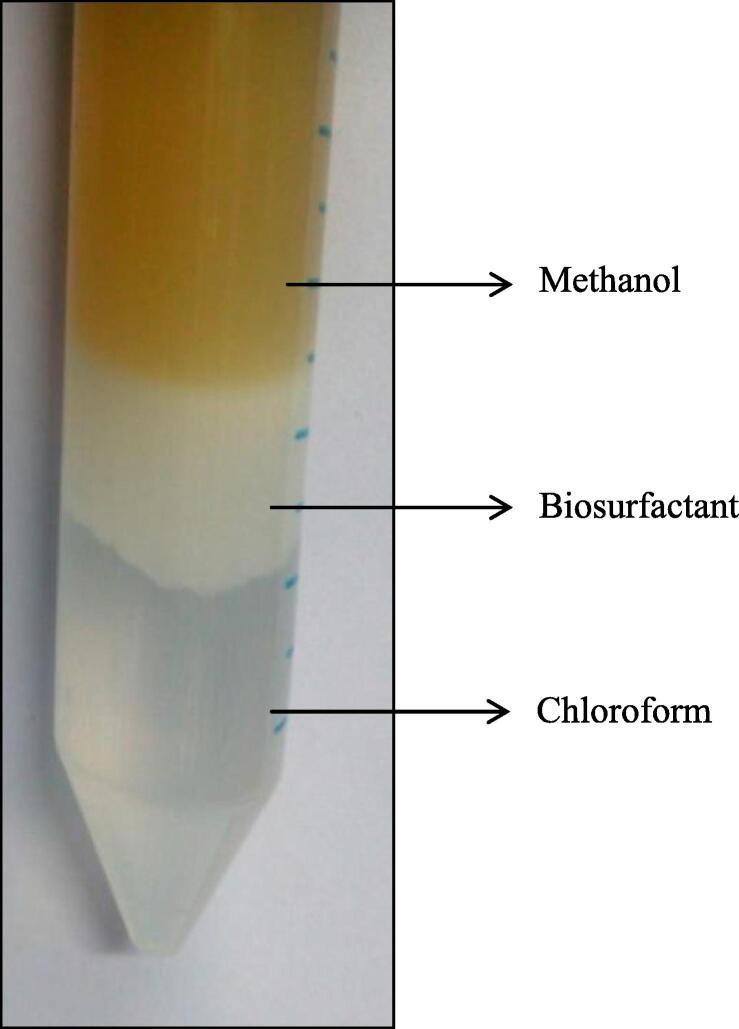
Fig. 1 shows the biosurfactant extracted from the cassava solid waste (production medium) using solvent extraction method in the period of 7 days. Based on the density difference, the chloroform was separated in the lower phase, methanol and the water were separated in the upper phase. The concentration of crude biosurfactant was found to be 0.34 mg/ml.
Fig. 1.
Extraction of Biosurfactant.
3.2. Construction of sand pack column
Sand Packed column was constructed using 35 cm × 2 cm glass column. The sterilized sand was packed tightly to obtain uniform packing (Fig. 2). Then the column was saturated with 5% NaCl solution. Then crude oil was added to it to study the efficiency of ex-situ enhanced oil recovery using biosurfactant and biopolymer incubation followed by brine flooding and then with biopolymer flooding after biosurfactant incubation (Sveistrup et al., 2016, Dhanarajan et al., 2017). The working of the packed column was studied.
Fig. 2.
Sand pack column. (a) Before oil incubation, (b) After oil incubation.
3.3. Ex-situ enhanced oil recovery
Ex-situ enhanced oil recovery was carried with the biosurfactant produced from Pseudomonas aeruginosa TEN01 and biopolymer (Chitosan). Numbers of experimental studies were done to optimise the amount of biosurfactant and biopolymer required to recover a maximum amount of entrapped oil (Dhanarajan et al., 2017).
3.3.1. Combined effect of biosurfactant and biopolymer incubation
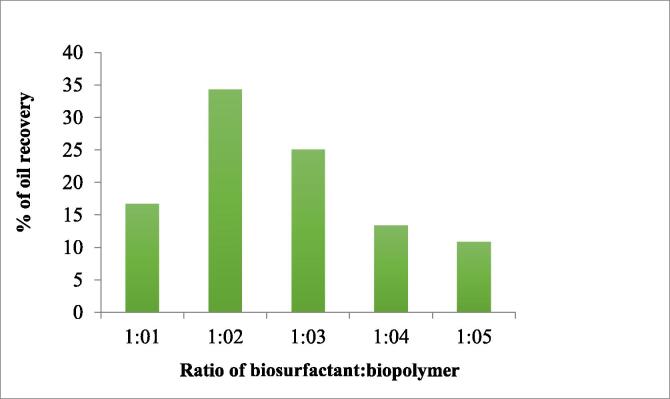
Soon after oil incubation, brine flooding was done to recover initial oil recovery. At the point when no more oil comes out, Biosurfactant and biopolymer were passed through the column and incubated for 24 h to recover the residual oil. Eftsoon, brine flooding was done to recover the released oil from the sand pack column. Here, biosurfactant ratio was kept constant and by varying biopolymer ratio for incubation, the oil recovery was estimated (Fig. 3).
Fig. 3.
Ex-situ enhanced oil recovery using Biosurfactant and biopolymer incubation.
Table 1 gives the general outcomes obtained in biosurfactant and biopolymer incubation for ex-situ enhanced oil recovery. Varying the ratio of the bioproducts, the oil recovery changes. While increasing the biopolymer ratio there was increment in oil recovery but during further rise in biopolymer ratio, the oil recovery decreased. It shows that optimal density of biopolymer also plays a role in oil recovery. Therefore at the ratio of 1:2 the density is optimum and hence higher yield of oil recovery. On increase in density of incubating solution by further increase in biopolymer solution, the recovery of oil decreased.
Table 1.
Ex-situ enhanced oil recovery with combined effect of biosurfactant and biopolymer.
| Trail no | Soi % | OOIP (ml) | Initial oil recovery |
Sor |
Biosurfactant: biopolymer ratio | AOR |
|||
|---|---|---|---|---|---|---|---|---|---|
| (ml) | % | (ml) | % | (ml) | % | ||||
| 1 | 45.84 | 11 | 5 | 45.45 | 6 | 54.54 | 1:1 | 1 | 16.66 |
| 2 | 60 | 8 | 4.5 | 56.25 | 3.5 | 43.75 | 1:2 | 1.2 | 34.28 |
| 3 | 50 | 10 | 6 | 60 | 4 | 40 | 1:3 | 1 | 25 |
| 4 | 40 | 15 | 9 | 60 | 6 | 40 | 1:4 | 0.8 | 13.33 |
| 5 | 58.33 | 14 | 8 | 57.2 | 6 | 42.8 | 1:5 | 0.6 | 10.8 |
Soi - Initial oil saturation.
OOIP - Original Oil In Place.
Sor - Residual oil saturation.
AOR - Additional Oil Recovery.
3.3.2. Flooding effect of biopolymer (Chitosan)
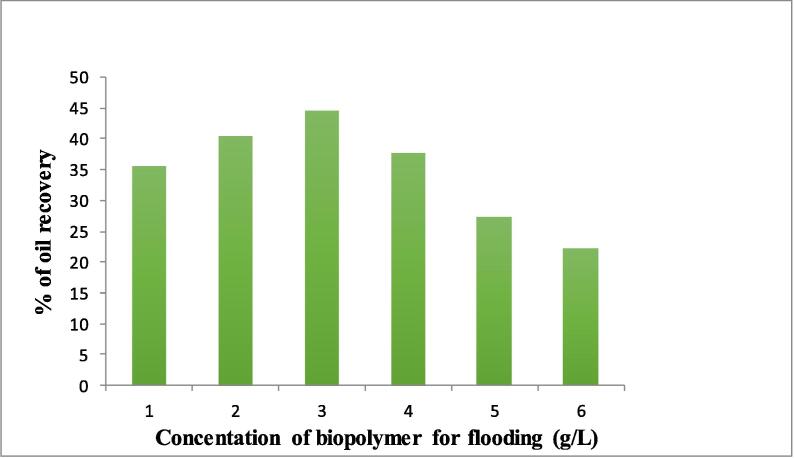
After oil incubation, brine flooding was done to obtain initial oil recovery (Fig. 4). When no more oil comes out, Biosurfactant was passed into the column (Lang and Wullbrandt, 1999) and incubated for 24 h. Then various concentration of biopolymer flooding was done to recover the released oil from the sand pack column (Dhanarajan et al., 2017).
Fig. 4.
Ex-situ enhanced oil recovery using Biosurfactant incubation and biopolymer flooding.
Table 2 gives the complete results of ex-situ enhanced oil recovery using Biosurfactant incubation and biopolymer flooding to retrieve the entrapped oil in the sand pack column. When the concentration of biopolymer was altered, there was increase in oil recovery to some extend after which it decreases. It shows density played a major role in these set of experiments also. There was higher oil recovery of 44.5% when flooding with biopolymer at the concentration of 3 g/L. While increasing the concentration after 3 g/L there was decrease in oil recovery (Dhanarajan et al., 2017).
Table 2.
Ex-situ enhanced oil recovery with combined effect of biosurfactant incubation and biopolymer flooding.
| Trail no | Soi % | OOIP (ml) | Initial oil recovery % | Sor % | Biopolymer flooding (g/L) | AOR |
||
|---|---|---|---|---|---|---|---|---|
| (ml) | % | |||||||
| 1 | 65.21 | 15 | 53.34 | 46.66 | Biosurfactant incubation (0.5 mg/ml) | 1 | 2.5 | 35.7 |
| 2 | 69.56 | 16 | 50.62 | 49.38 | 2 | 3.2 | 40.5 | |
| 3 | 69.56 | 16 | 50 | 50 | 3 | 3.56 | 44.5 | |
| 4 | 60.86 | 14 | 50.72 | 49.88 | 4 | 2.6 | 37.68 | |
| 5 | 65.21 | 15 | 51.34 | 48.66 | 5 | 2.0 | 27.3 | |
| 6 | 69.56 | 16 | 49.37 | 50.63 | 6 | 1.8 | 22.22 | |
Soi - Initial oil saturation.
OOIP - Original Oil In Place.
Sor - Residual oil saturation.
AOR - Additional Oil Recovery.
Comparative study between mere biosurfactant-biopolymer incubation with brine flooding in sand pack column and biosurfactant incubation with biopolymer flooding in sand pack column showed that the later case gave better result. That was 44.5% of oil recovery was obtained in biopolymer flooding whereas 34.28% only was obtained in biosurfactant- biopolymer incubation. Higher oil recovery was seen in the concentration of 3 g/L of biopolymer flooding. In case of biosurfactant- biopolymer incubation, oil recovery was more in the ratio of 1:2 (Fig. 5). The percentage of increase in oil recovery may be due to the optimum density and viscosity of the biopolymer because in both the cases, the concentration of biosurfactant was kept constant (Lazar et al., 2007, Patel et al., 2015, Sen, 2008).
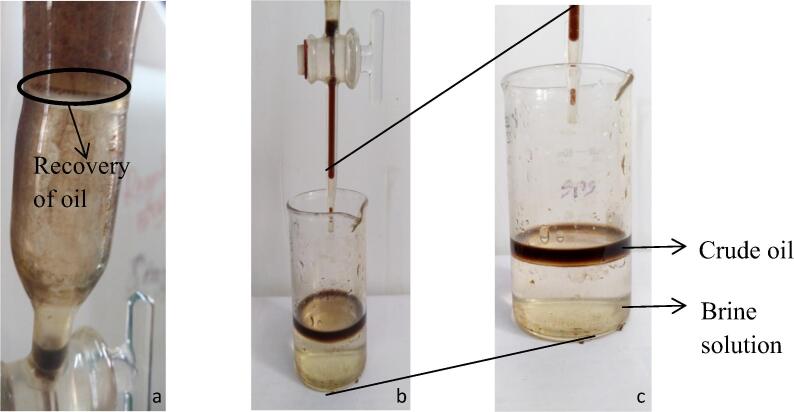
Fig. 5.
Oil recovery. (a) Recovery of oil, (b) collection of recovered oil, (c) separation of crude oil and brine solution after recovery.
3.4. UV–Visible spectrum of Ag NPS
The UV–Vis spectra (Fig. 6) of the samples also confirms the formation of silver nanoparticles. We can observe the λmax at 423 nm (Fig. 6a) while using SDS and 428 nm (Fig. 6b) while using biosurfactant (Das et al., 2016).
Fig. 6.
UV–Visible spectra of Ag NPs formed by (A) SDS, (B) Biosurfactant.
3.5. XRD analysis of Ag NPs
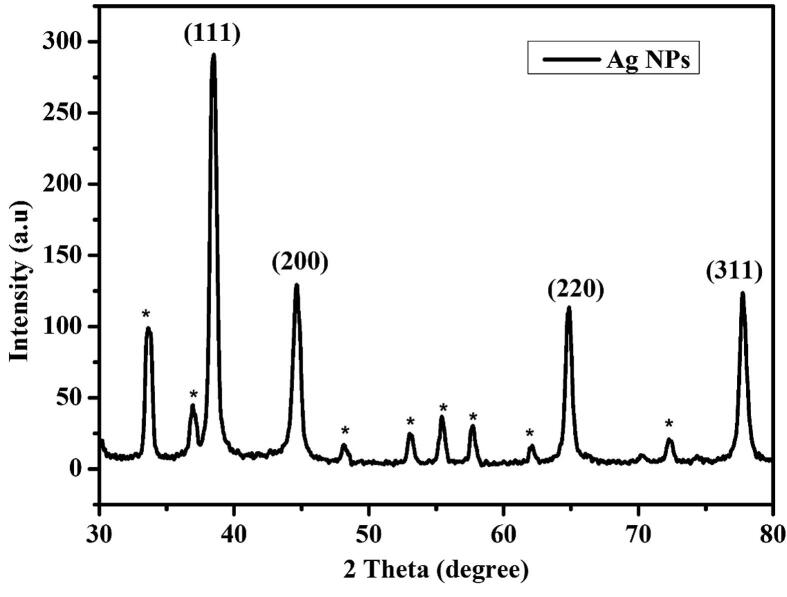
Crystalline size analysis was carried for the synthesized silver nanoparticles. The diffraction peak of 2θ = 38.5°, 44.4°, 64.6° and 77.5°, plane of (1 1 1), (2 0 0), (2 2 0) and (3 1 1) of particular indicates the Ag NPs are cubic structure is compared with JCPDS no: 01-087-0720 (Fig. 7). The peaks are confirmed Ag nanoparticles were highly crystalline. The average sizes are calculated by Debye Scherrer formula. A few peaks (Star sign) are unassigned were also observed suggesting that crystalline of bio-organic phase occur on the surface of the Ag NPs (De Almeida et al., 2016).
Fig. 7.
Structural analysis of Ag NPs.
3.6. Morphology analysis of Ag NPs
The SEM images of the Ag NPs are shown in Fig. 8. which depicts the products possess high density zero dimensional structures with diameter range of 30–150 nm, also random distribution of silver particles in the samples were noticed. Upon careful observation, the micrograph depicts agglomerated particles and porous islands (Kamal et al., 2017).
Fig. 8.
SEM image of Ag NPs.
3.7. Particle size and stability of Ag NPs
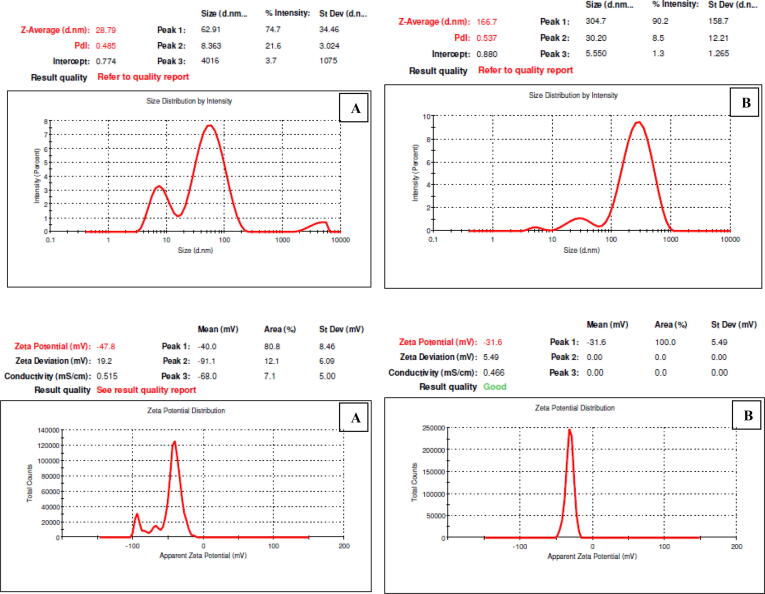
The Fig. 9 shows, the Z-Average size of the particles synthesized using (A) SDS and (B) biosurfactant was determined as 28.79 nm and 166.7 nm respectively which confirmed that the particles are in nanosize. The stability of the nanoparticles synthesized by (A) SDS and (B) biosurfactant was also determined by zeta potential values −47.8 mV and −31.6 mV respectively. By referring to Das et al. (2016), we can conclude that the nanoparticles synthesized using biosurfactant have higher stability than the particles synthesized using SDS.
Fig. 9.
Zeta Size Distribution & Zeta potential of (A) SDS based Ag NPs (B) Biosurfactant based Ag NPs.
3.8. Sand pack column result for biosurfactant based nanoparticle
The Sand pack column was constructed and operated by above mentioned procedure. The mechanism of sand pack column was observed and the pore volume of sand pack column was found to be 25 ml which is the volume of brine required to saturate the column (Ogolo et al., 2012).
3.9. Percentage efficiency of oil recovery
| (1) | By using CS-AgNPs |
|---|---|
| Pore volume | = 25 ml |
| Initial oil volume | = 20 ml |
| Residual oil volume | = 10 ml |
| Volume of AgNPs | = 10 ml |
| Volume of oil recovered after | |
| SDS based AgNPs flooding | |
| Oil recovery % | = (1.3/8.7) × 100 |
| Percentage of Oil recovery | = 14.94% |
| (2) | By using BS-AgNPs |
| Pore volume | = 25 ml |
| Initial oil volume | = 15 ml |
| Residual oil volume | = 8 ml |
| Volume of AgNPs | = 10 ml |
| Volume of oil recovered after | |
| BS-AgNPs flooding | |
| Oil recovery % | = (1/7) × 100 |
| Percentage of Oil recovery | = 14.28% |
In the first case, we used chemically synthesized Ag NPs and we recovered 1.3 ml of oil from 10 ml and in the second, we used biosurfactant based Ag Nps and we recovered 1 ml of oil from 8 ml. From the results, we can observe that both chemical based AgNPs and biosurfactant based AgNPs shows similar efficiency in EOR.
4. Conclusion
Enhanced oil recovery was done using the bioproducts such as Biosurfactant (Rhamnolipid), biopolymer (Chitosan) and Biosurfactant based nanoparticle (Silver nanoparticle). The oil recovery in combined incubation of Biosurfactant- biopolymer and brine flooding was found to be 34.28% whereas for Biosurfactant incubation and biopolymer flooding was 44.5%. Biosurfactant based silver nanoparticle produced has higher stability and the oil recovery was found to be 14.28% which was similar to that of oil recovery obtained via chemically produced silver nanoparticle. From the results obtained so far, flooding with biopolymer after Biosurfactant incubation gave better yield of oil recovery. However, the nanoparticle also enhanced the oil recovery by electrostatic repulsive interaction between NP and Biosurfactant (Kamal et al., 2017). But the results were not satisfying when compared to biopolymer flooding after Biosurfactant incubation as that has 3 fold higher oil recovery. Hence it is concluded that Biosurfactant is general enhancer for oil recovery but with addition to biopolymer it does noteworthy jobs in recovering oil entrapped within sand. On considering biopolymer as incubating and flooding agent, their densifying and viscosifing activities enhanced the oil recovery. This higher density fluid helps blocking their zones in case of incubating agent and sweeping out oil for the case of flooding solution. Hence, Biosurfactant (Rhamnolipid) and biopolymer (chitosan) can be used in EOR for better oil recovery in tertiary stage.
Acknowledgement
The authors gratefully acknowledge the support from Department of Biotechnology, Anna University BIT campus, Trichy, Tamil Nadu, India. The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through Research Group No. RG-1438- 091.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Periyasamy SureshKumar, Email: drsureshbiotech2003@gmail.com.
Marimuthu Govindarajan, Email: drgovind1979@gmail.com.
References
- Abdel M.A.M., Lepine F., Deziel E. Rhamnolipids: diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010;86(5):1323–1336. doi: 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sulaimani H., Joshi S., Al-Wahaibi Y., Al-Bahry S., Elshafie A., Al-Bemani A. Microbial biotechnology for enhancing oil recovery: current developments and future prospects. Biotechnol. Bioinform. Bioeng. 2011;1:147–158. [Google Scholar]
- Al-Wahaibi Y., Al-Hadrami H., Al-Bahry S., Elshafie A., Al-Bemani A., Joshi S. Injection of biosurfactant and chemical surfactant following hot water injection to enhance heavy oil recovery. Pet. Sci. 2016;13:100–109. [Google Scholar]
- Amani H., Mehrnia M.R., Sarrafzadeh M.H., Haghighi M., Soudi M.R. Scale up and application of biosurfactant from Bacillus subtilis in enhanced oil recovery. Biotechnol. Appl. Biochem. 2010;162(2):510–523. doi: 10.1007/s12010-009-8889-0. [DOI] [PubMed] [Google Scholar]
- Amani H., Muller M.M., Syldatk C., Hausmann R. Production of microbial rhamnolipid by Pseudomonas Aeruginosa MM1011 for ex situ enhanced oil recovery. Biotechnol. Appl. Biochem. 2013;70(5):1080–1093. doi: 10.1007/s12010-013-0249-4. [DOI] [PubMed] [Google Scholar]
- Bachmann R.T., Johnson A.C., Edyvean R.G.J. Biotechnology in the petroleum industry: an overview. Int. Biodeterior. Biodegrad. 2014;86:225–237. [Google Scholar]
- Banat I.M. Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: a review. Bioresour. Technol. 1995;51:1–12. [Google Scholar]
- Das M., Patowary K., Vidya R., Malipeddi H. Microemulsion synthesis of silver nanoparticles using biosurfactant extracted from Pseudomonas aeruginosa MKVIT3 strain and Comparison of their antimicrobial and cytotoxic activities. IET Nanobiotechnol. 2016;10(6):411–418. doi: 10.1049/iet-nbt.2015.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A.D.G., Soares Da Silva R.C.F., Luna J.M., Rufino R.D., Santos V.A., Banat I.M., Sarubbo L.A. Biosurfactants: promising molecules for petroleum biotechnology advances. Front Microbiol. 2016;7:1718. doi: 10.3389/fmicb.2016.01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai J.D., Banat I.M. Microbial production of surfactants and their commercial potential. Mol. Bio. Rev. 1997;61:47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanarajan G., Rangarajan V., Bandi C., Dixit A., Das S., Ale K., Sen R. Biosurfactant-biopolymer driven microbial enhanced oil recovery (MEOR) and its optimization by an ANN-GA hybrid technique. J. Biotechnol. 2017;256:46–56. doi: 10.1016/j.jbiotec.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Elraies K.A., Tan I.M. The application of a new polymeric surfactant for chemical EOR. In: Romero-Zerón L., editor. Introduction to Enhanced Oil Recovery (EOR) Processes and Bioremediation of Oil-contaminated Sites. InTech; Rijeka: 2012. pp. 45–70. [Google Scholar]
- Falatko D.M. Virginia Polytechnic Institute and State University; Blackburg, VA: 1991. Effects of biologically reduced surfactants on the mobility and biodegradation of petroleum hydrocarbons. M.S. Thesis. [Google Scholar]
- Gao C. Application of a novel biopolymer to enhance oil recovery. J. Petrol. Explor. Prod. Technol. 2016;6(4):749–753. [Google Scholar]
- Gao C.H., Zekri A. Applications of microbial-enhanced oil recovery technology in the past decade. Energ Source Part A. 2011;33:972–989. [Google Scholar]
- Geetha S.J., Banat I.M., Joshi S.J. Biosurfactants: Production and potential applications in microbial enhanced oil recovery (MEOR) Biocatal. Agricu. Biotech. 2018;14:23–32. [Google Scholar]
- Gogoi S.B. Adsorption-desorption of surfactant for enhanced oil recovery. Transp. Porous Med. 2011;90:589–604. [Google Scholar]
- Guo K., Li H., Yu Z. Metallic nanoparticles for enhanced heavy oil recovery: promises and challenges. Energy Procedia. 2015;75:2068–2073. [Google Scholar]
- Jarvis F.G., Johnson M.J. A Glyco-lipid Produced by Pseudomonas Aeruginosa. J. Am. Chem. Soc. 1949;71(12):4124–4126. [Google Scholar]
- Jha A.C. Heavy-oil recovery from thin pay zones by electromagnetic heating'. Energy Sources. 1999;21(1–2):63–73. [Google Scholar]
- Joshi S.J., Abed R.M.M. Biodegradation of polyacrylamide and its derivatives. Environ. Process. 2017;4:463–476. [Google Scholar]
- Kamal M.S., Adewunmi A.A., Sultan A.S., Al-Hamad M.F., Mehmood U. Recent advances in nanoparticles enhanced oil recovery: rheology, interfacial tension, oil recovery and wettability alteration. J. Nanomater. 2017 [Google Scholar]
- Kaskatepe B., Yildiz S., Gumustas M., Ozkan S.A. Biosurfactant production by P. aeruginosa in kefir and fish meal. Braz. J Microbiol. 2015;46(3):855–859. doi: 10.1590/S1517-838246320140727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S., Wullbrandt D. Rhamnose lipids - biosynthesis, microbial production and application potential. Appl. Microbiol. Biotechnol. 1999;51(1):22–32. doi: 10.1007/s002530051358. [DOI] [PubMed] [Google Scholar]
- Lazar I., Petrisor I., Yen T. Microbial enhanced oil recovery (MEOR) Pet. Sci. Technol. 2007;25:1353–1366. [Google Scholar]
- Levitt D., Jackson A., Heinson C., Britton L.N., Malik T., Dwarakanath V., Pope G.A. SPE/DOE Symposium on Improved Oil Recovery. Society of Petroleum Engineers; 2006. Identification and evaluation of high-performance EOR surfactants. [Google Scholar]
- Li L., Yuan X., Sun J., Xu X., Li S., Wang L. International Petroleum Technology Conference, Beijing. 2013. Vital role of nanotechnology and nanomaterials in the field of oilfield chemistry. [Google Scholar]
- Li Q., Kang C., Wang H., Liu C., Zhang C. Application of microbial enhanced oil recovery to Daqing Oilfield. Biochem. Eng. J. 2002;3593:1–3. [Google Scholar]
- Loria H., Trujillo F.G., Sosa S.C., Pereira A.P. Kinetic modeling of bitumen hydroprocessing at in-reservoir conditions employing ultra-dispersed catalysts. Energ Fuel. 2011;25:1364–1372. [Google Scholar]
- McInerney M.J., Nagle D.P., Knapp R.M. Microbially enhanced oil recovery: past, present, and future. Petrol. Microbiol. 2005:215–237. [Google Scholar]
- Mohsenzadeh A., Al-Wahaibi Y., Al-Hajri R., Jibril B., Joshi S., Pracejus B. Investigation of formation damage by Deep Eutectic Solvents as new EOR agents. J. Pet. Sci. Eng. 2015;129:130–136. [Google Scholar]
- Mulligan C.N., Gibbs B.F. Factors influencing the economics of biosurfactants; in biosurfactants, properties and applications. Surfactant Sci. 1993;48:329–371. ed. N Kosaric., 48, 329-371. [Google Scholar]
- Nitschke M., Maria G. Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava waste water. Bioresour. Technol. 2006;97:336–341. doi: 10.1016/j.biortech.2005.02.044. [DOI] [PubMed] [Google Scholar]
- Ogolo N.A., Olafuyi O.A., Onyekonwu M.O. SPE Saudi Arabia Section Technical Symposium and Exhibition. Society of Petroleum Engineers; 2012. Enhanced oil recovery using nanoparticles. [Google Scholar]
- Patel J., Borgohain S., Kumar M., Rangarajan V., Somasundaran P., Sen R. Recent developments in microbial enhanced oil recovery. Renew. Sust. Energ. Rev. 2015;52:1539–1558. [Google Scholar]
- Pornsunthorntawee O., Arttaweeporn N., Paisanjit S., Somboonthanate P., Abe M., Rujiravanit R., Chavadej S. Isolation and comparision of biosurfactants produced by Bacillus subtilis PT2 and Pseudomonas aeruginosa SP4 for microbial surfactant-enhanced oil recovery’. Biochem. Eng. J. 2008;42:172–179. [Google Scholar]
- Rahman K.S.M., Gakpe E. Production, characterization and applications of biosurfactants. Review. Biotechnol. 2008;7:360–370. [Google Scholar]
- Reddy A.S., Chen Y.C., Simon C.B., Chien C.C., Jiin S.J., Cheng W.F., Hau R.C., Jung C.W. Synthesis of silver nanoparticles using surfactin: a biosurfactant as stabilizing agent. Mater. Lett. 2009;63:1227–1230. [Google Scholar]
- Rudyk S., Spirov P., Samuel P., Joshi S.J. Vaporization of crude oil by supercritical CO2 at different temperatures and pressures: example from Gorm field in the Danish North Sea. Energy Fuels. 2017;31:6274–6283. [Google Scholar]
- Schulz R. The reason to expect prolonged USD 30–60/Bbl oil. J. Petrol. Technol. 2016;68:42–45. [Google Scholar]
- Sen R. Biotechnology in petroleum recovery: the microbial EOR. Prog. Energy Combust. Sci. 2008;34(6):714–724. [Google Scholar]
- Shah D.O. first ed. Elsevier Academic Press; 2012. Improved Oil Recovery by Surfactant and Polymer Flooding. [Google Scholar]
- Silva R.D.C.F., Almeida D.G., Rufino R.D., Luna J.M., Santos V.A., Sarubbo L.A. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int. J. Mol. Sci. 2014;15:12523–12542. doi: 10.3390/ijms150712523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simjoo M., Rezaei M.A., Nadri F., Mousapour M.S., Iravani M., Chahardowli M. IOR 2019–20th European Symposium on Improved Oil Recovery. 2019. Introducing a new, low-cost biosurfactant for EOR applications: a mechanistic study. [Google Scholar]
- Sivasubramani A., Selvaraj R. Isolation, Screening and Production of Biosurfactant by P. aeruginosa SD4 using various hydrocarbon sources. IJSR. 2017;6(2):2319–7064. [Google Scholar]
- Stosur G.J. Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific. Society of Petroleum Engineers; 2003. EOR: Past, present and what the next 25 years may bring. [Google Scholar]
- Suthar H., Hingurao K., Desai A., Nerurkar A. Evaluation of Bioemulsifier mediated Microbial Enhanced Oil Recovery using sand pack column. J. Microbiol. Meth. 2008;75:225–230. doi: 10.1016/j.mimet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Sveistrup M., Van Mastrigt F., Norrman J., Picchioni F., Paso K. Viability of biopolymers for enhanced oil recovery. J. Disper. Sci. Technol. 2016;37(8):1160–1169. [Google Scholar]
- Zhao F., Shi R., Zhao J., Li G., Bai X., Han S., Zhang Y. Heterologous production of Pseudomonas aeruginosa rhamnolipid under anaerobic conditions for microbial enhanced oil recovery. J. Appl. Microbiol. 2015;118(2):379–389. doi: 10.1111/jam.12698. [DOI] [PubMed] [Google Scholar]