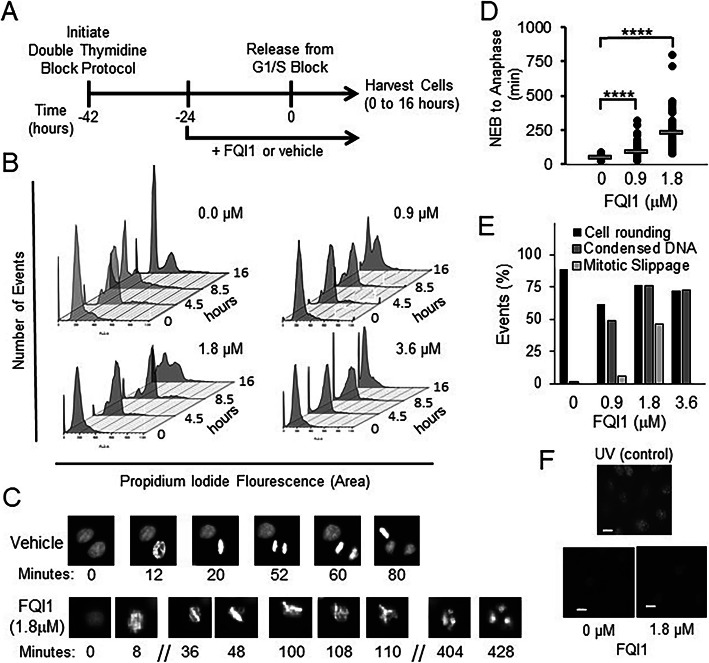
Fig. 1.
FQI1-treated HeLa cells exhibit mitotic defects. a Schematic of experimental protocol. Cells released from the double thymidine block in the presence of 20 μM of thymidine, plus FQI1 or vehicle, were harvested at multiple times during progression through the cell cycle. b At the indicated time points following release from the G1/S block with 0, 0.9, 1.8, or 3.6 μM of FQI1, cells were analyzed for DNA profiling by flow cytometry. Data are representative of at least three independent experiments. Separated, individual flow cytometry images are displayed in Additional File 1. c Representative time-lapse images of individual cells treated with vehicle or 1.8 μM FQI1. Numbers represent time for one particular cell in the image from nuclear envelope breakdown (designated as time = 0 for that cell). d Quantitation of mitotic time from nuclear envelop breakdown (NEB) to anaphase for the population of asynchronous cells during treatment for approximately 16 h with FQI1 or vehicle. Mitotic times (mean time in minutes +/− standard error of the mean, n) for vehicle, and 0.9 or 1.8 μM FQI1 treatments were: 48.7 +/− 1.5, 104; 84.5 +/− 4.9, 104; and 228 +/− 15, 77; respectively. Mitotic time for cells treated with 3.6 μM was not quantifiable, as those cells that entered mitosis at various points during the imaging period never reached anaphase or nuclear division by the end of the 16-h period. e Quantitation of cellular events at increasing concentrations of FQI1 during time lapse microscopy, including percentage of cells that visually rounded up as expected for mitotic entry (by phase contrast), but were delayed with condensed, but unaligned chromosomes, and the percentage that apparently underwent mitotic slippage with formation of multiple (> 2) nuclei. 120–140 cells were analyzed for each condition. f Bottom: γ-H2AX staining of HeLa cells treated with vehicle or 1.8 μM FQI1. Top: Representative image of UV-treated HeLa cells as a positive control. All images were taken at the same intensity and are representative of two independent experiments. Scale bars: 20 μm