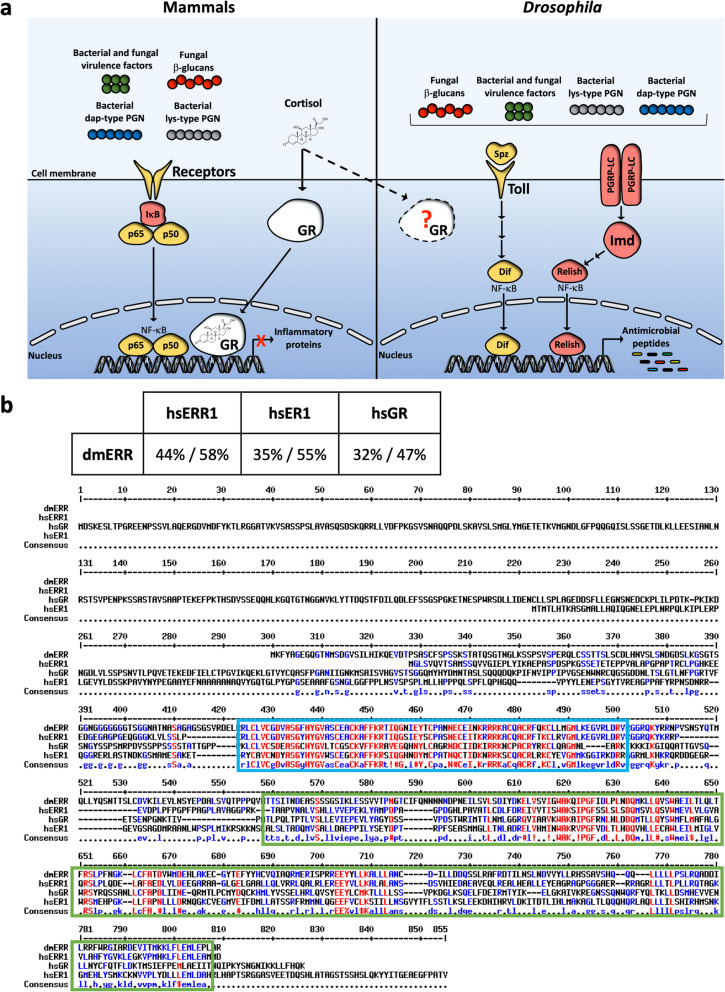
Fig. 1.
Identification of GR sequence-homolog in Drosophila melanogaster. a An illustration of the hypothesis for the existence of GR ortholog in fruit flies. During mammalian microbial infection (left panel), cell surface receptors, such as Toll-like receptors, detect bacterial and fungal cells. Once bound to microbial cells, mammalian receptors activate the expression of pro-inflammatory genes through the actions of Nuclear Factor κB (NF-κB). Steroids, such as cortisol, binds to a nuclear receptor, glucocorticoid receptor (GR), and trigger its translocation into the nucleus, where it represses NF-κB and the expression of pro-inflammatory genes. Similar pathways exist in insects (right panel), where two pathways detect invading microbial pathogens, and ultimately trigger the expression of antimicrobial peptides. Toll and Imd pathways both ultimately activate NF-κB transcription factors, which induce the transcription of antimicrobial peptides. We hypothesize the existence of a GR ortholog in fruit flies, capable of immunosuppressing infected insects in response to steroids. b Multiple amino acid sequence alignment between D. melanogaster Estrogen Related Receptor (dmERR), Homo sapiens ERR (hsERR1), H. sapiens estrogen receptor (hsER1), and H. sapiens glucocorticoid receptor (hsGR). The sequences of dmERR (Accession NP_648183), hsERR1 (Accession XP_016872802), hsER1 (Accession XP_016865870), and hsGR (Accession CAJ65924) were aligned using MultAlin software. The DNA binding domain and ligand binding domain are highlighted in blue and green boxes, respectively. Identical amino acids are shown in red. The extent (%) of the identity/similarity between Drosophila and human sequences is shown above the alignment