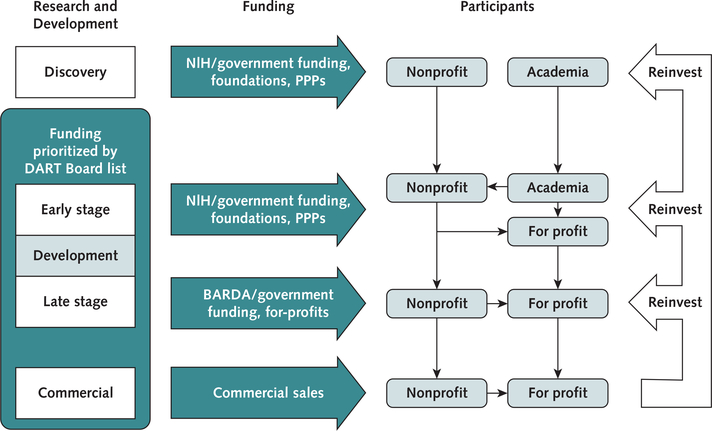
Figure.1. Schematic of a sustainable model for targeted discovery and development of new antibiotics.
Stages include Discovery (basic science work and lead-candidate identification), Early Development (lead optimization, manufacturing, preclinical toxicity, pharmacology, IND preparation, and phase I clinical trials), Late Development (phase II/III clinical trials and NDA filing), and Commercialization (postmarketing). Prioritization of funding to target clinical unmet needs would be set by the DART Board list from Early Development through Commercialization, with the ability to influence the Discovery phase. Academia will continue to conduct early disovery work using existing funding mechanisms. Nonprofits would participate at all stages. For-profit companies could continue to participate at all stages, particularly via outlicensing arrangements with academia and nonprofits. Revenue from commercial sales and licensing fees from for-profit companies would be fed back into nonprofits and/or government funding agencies whose mission is to discover and develop new antibiotics. BARDA = Biomedical Advanced Research and Development Authority; DART = Developing Antibiotics for Resistant Targets; IND = Investigational New Drug; NDA = New Drug Application; NIH = National Institutes of Health; PPPs = public private partnerships.