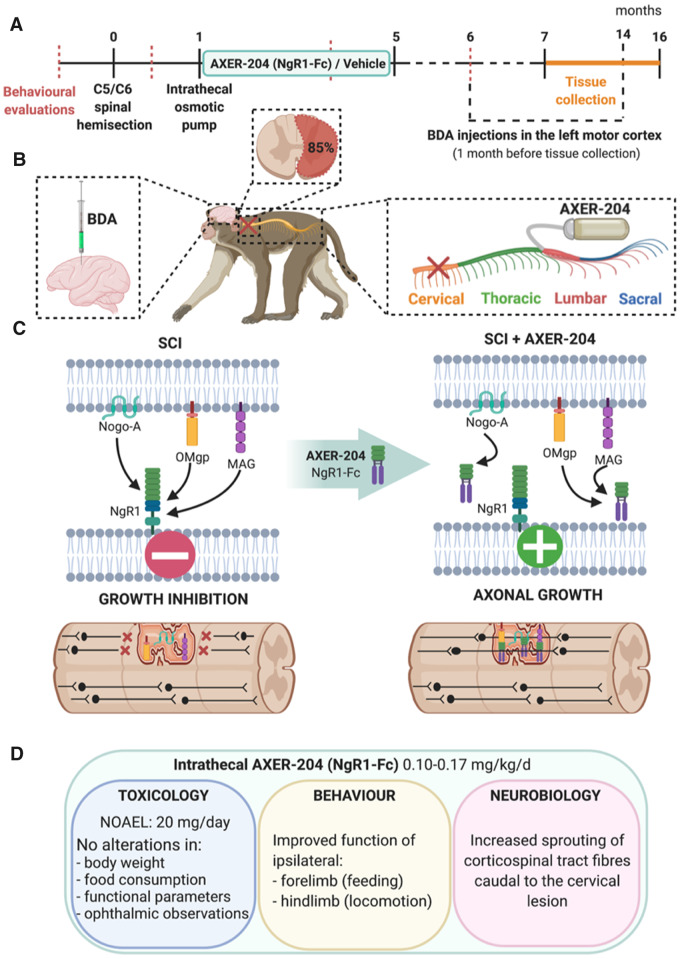
Figure 1.
Schematic of experimental design and key findings. (A) Timeline of the experimental protocol showing time points of behavioural evaluation, spinal cord hemisection injury, delivery of AXER-204 (NgR1-Fc) or vehicle over 4 months, biotinylated dextran amine (BDA) tracer injections and tissue collection between 7 and 16 months after injury. (B) Schematic representation of surgical protocols performed in African green monkeys, depicting the unilateral hemisection injury at cervical level C5/C6, intrathecal catheter implantation at the lumbar level for continuous infusion of the drug via a connected minipump and BDA injections into the left motor cortex to label descending axons of the corticospinal tract. (C) Illustration of molecular events occurring after spinal cord injury and in response to treatment with AXER-204. Following spinal cord injury (SCI), myelin-associated neuronal growth inhibitors such as Nogo-A, myelin-associated glycoprotein (MAG) and oligodendrocyte myelin glycoprotein (OMgp) are intensely expressed and bind to the Nogo-66 receptor 1 (NgR1), causing growth cone collapse and inhibiting neurite outgrowth. Intrathecal treatment with AXER-204, the Nogo receptor decoy, traps these myelin-associated growth inhibitors, effectively blocking NgR1 signalling, which enables axonal growth and neuroplasticity to occur within the normally inhibitory spinal cord injury environment. (D) AXER-204 delivered intrathecally to non-human primates with cervical level spinal cord injuries has a favourable toxicology profile, promotes recovery of forelimb function during feeding and hindlimb locomotor function in the open field, and enables regeneration of the corticospinal tract, a major descending motor pathway important for skilled voluntary control. NOAEL = no observed adverse effect level. Image created with BioRender.com.