Abstract
In the summer of 2017, 4 horses were diagnosed with septic fibrinous pericarditis at the Western College of Veterinary Medicine, Saskatoon. This case series occurred after a significant outbreak of forest tent caterpillars (Malacosoma disstria) in the province during that spring. Three horses were immediately euthanized, and treatment was attempted in 1 mare. This is the first case series of pericarditis possibly associated with the ingestion of forest tent caterpillars to be reported in western Canada. Although cause-effect is not proven, it is prudent to prevent the ingestion of caterpillars by horses.
Key clinical message:
Septic fibrinous pericarditis, a rare condition in horses, has previously been linked to outbreaks of eastern tent caterpillars. A similar link might exist in this case series.
Résumé
Péricardites fibrineuses septiques chez quatre chevaux saskatchewanais consécutive à une infestation de chenilles de livrée des forêts en 2017. Au cours de l’été 2017, quatre chevaux ont été diagnostiqués avec une péricardite fibrineuse septique au Western College of Veterinary Medicine de Saskatoon. Ces cas ont été présentés après une sévère infestation printanière de chenilles de livrée de forêts (Malacosoma disstria) dans la province de la Saskatchewan, reportée au printemps. Trois chevaux ont été immédiatement euthanasiés et une jument a été hospitalisée pour traitement. Ceci est le premier rapport décrivant la possible association entre des cas de péricardite chez des chevaux et l’ingestion de chenilles de livrée de forêts dans l’Ouest canadien. Même si le lien de cause à effet n’est pas prouvé, il est prudent d’éviter l’ingestion de ces chenilles processionnaires par les chevaux.
Message clinique clé :
La péricardite fibrineuse septique, une condition rare chez les chevaux, a précédemment été liée à des flambées de livrées des forêts. Un lien similaire pourrait exister dans la présente série de cas.
(Traduit par les auteurs)
Case descriptions
In the spring of 2017, Saskatchewan experienced an outbreak of forest tent caterpillars (Malacosoma disstria) (1). That summer, 4 horses from Saskatchewan were presented to the Western College of Veterinary Medicine (WCVM), University of Saskatchewan, and were diagnosed with right-sided congestive heart failure due to septic fibrinous pericarditis. All horses were housed on pasture with other apparently healthy horses. According to the owners, all 4 horses had their environment infested by forest tent caterpillars within the past 2 springs. It was suspected that they ingested caterpillars as previously reported (2).
Case 1
A 3-year-old Quarter Horse stallion was referred at the end of June for evaluation of suspected pleuropneumonia of 11 days’ duration. He had been bought as a yearling and had not received any vaccines since he was purchased but was dewormed that spring (unknown product). No previous medical history was reported. The horse was initially treated by the referring veterinarian for lethargy and fever with 5 d of penicillin switched to ceftiofur and phenylbutazone (unknown products and dosages) until the day before presentation when the horse was deteriorating, developing ventral edema, signs of respiratory difficulty, and colic. On presentation, muffled heart sounds led to the suspicion of pericardial effusion; the jugular vein distension, weak peripheral pulse, and pitting edema led to the suspicion of right-sided constrictive cardiomyopathy. These cardiovascular abnormalities suggested right-sided congestive heart failure. The abnormalities of the respiratory system associated with decreased lung field auscultation led to the suspicion of pleural effusion (Tables 1 and 2), which could also have contributed to muffle the heart sounds. Ultrasonographic examination was consistent with tri-cavitary effusion (pericardial, pleural, and peritoneal) with fibrinous pericarditis (Figure 1). Results from venous blood gas, electrolytes and L-lactate, were overall unremarkable. The horse was euthanized due to financial constraints and grave prognosis.
Table 1.
Clinical findings obtained at presentation of 4 horses with septic fibrinous pericarditis referred to the Western College of Veterinary Medicine, University of Saskatchewan, between June and September 2017, following an environmental infestation with forest tent caterpillars. Abnormalities are highlighted in bold.
| Case 1 | Case 2 | Case 3 | Case 4 | Normal values | |
|---|---|---|---|---|---|
| Rectal temperature | |||||
| °C | 38.1 | 38.8 | 38.7 | 36.4 | 37.2 to 38.5 |
| °F | 100.5 | 101.8 | 101.6 | 97.5 | 98.9 to 101.3 |
| Heart rate/pulse (beats/min) | 68a | 76 | 60a | 52a | 26 to 44 |
| Respiratory rate (breaths/min) | 36 | 34 | 28 | 20 | 8 to 16 |
The heart rate/pulse was assessed by palpation of the facial artery because heart sounds were not audible on auscultation.
Table 2.
Problem list following the physical examination of 4 horses with septic fibrinous pericarditis referred to the Western College of Veterinary Medicine, University of Saskatchewan, between June and September 2017, following an environmental infestation with forest tent caterpillars.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| General condition | Loss of body condition | Body condition score 4/9 | Loss of body condition | |
| Lethargy | Lethargy | Lethargy, anorexia | Lethargy, anorexia | |
| Hyperthermia | Hyperthermia | Hyperthermia | ||
| Colic | ||||
| Cardiovascular system | Tachycardia | Tachycardia | Tachycardia | Tachycardia |
| Muffled heart sounds | Muffled heart sounds | Muffled heart sounds | Muffled heart sounds | |
| Weak peripheral pulse | Weak peripheral pulse | |||
| Jugular vein distension | Jugular vein distension | Jugular vein distension | Jugular vein distension | |
| Retrograde jugular pulse | Retrograde jugular pulse | Retrograde jugular pulse | ||
| Ventral pitting edema | Ventral pitting edema | Ventral pitting edema | Ventral pitting edema | |
| Congested gingival mucous membranes | Congested gingival mucous membranes | |||
| Respiratory system | Tachypnea | Tachypnea | Tachypnea | Tachypnea |
| Nostril flaring | Nostril flaring | Nostril flaring | ||
| Dull thoracic percussion | Dull thoracic percussion | |||
| Decreased lung field auscultation | Decreased lung field auscultation | Decreased lung field auscultation | ||
| Pain reaction at palpation of intercostal spaces | Abdominal expiratory effort | |||
| Gastrointestinal system | Loose stool | Soft stool | Diarrhea | Soft stool |
| Hypermotile borborygmi | Decreased borborygmi |
Figure 1.
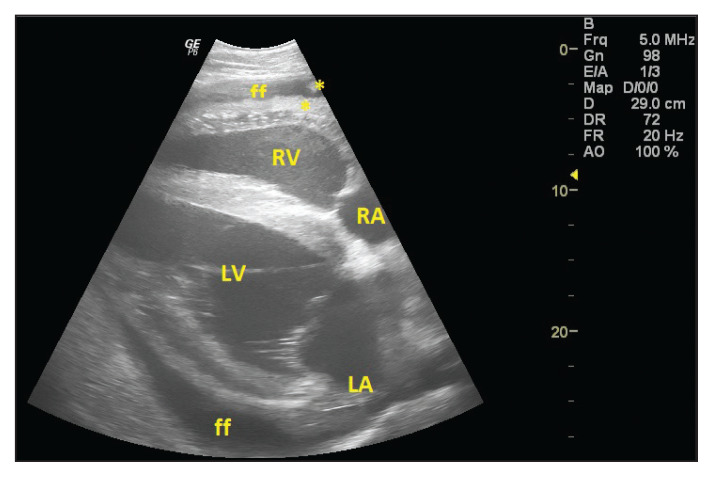
Right parasternal long-axis 4-chamber echocardiographic view of an 18-year-old Appaloosa mare (Case 3) diagnosed with fibrinous pericarditis. Dorsal is to the right. Villonodular fibrin (*) was adhering to the epicardium and visceral layer of pericardium, moderate pericardial effusion (ff) was present. RA — right atrium; LA — left atrium; RV — right ventricle; LV — left ventricle.
Case 2
An 8-year-old Quarter Horse gelding was presented 3 d after Case 1, for evaluation of lethargy, ongoing for a week. The horse had been owned for 3 y with no previous medical history, was up-to-date on vaccinations (unknown product) and dewormed with ivermectin paste in the spring and fall. Sheath edema was noticed a few days before presentation; the horse started to pass loose manure and ventral edema appeared the day of presentation. Penicillin (unknown dosage and time of administration) was administered by the owner. On presentation, right-sided congestive heart failure was suspected (Tables 1 and 2). Thoracic ultrasound was consistent with pleural effusion and fibrinous effusive pericarditis (Figure 1). Abdominal ultrasound revealed ascites and suggested edema of the mesentery. Venous blood gas was overall unremarkable except for electrolyte abnormalities, confirmed on the biochemistry panel, including hyponatremia [119.5 mmol/L; reference range (RR): 132 to 142 mmol/L], and hypochloremia (81 mmol/L; RR: 92 to 103 mmol/L). On biochemistry, the other relevant abnormalities included low total calcium (2.26 mmol/L; RR: 2.39 to 3.38 mmol/L), hyperphosphatemia (1.93 mmol/L; RR: 0.53 to 1.19 mmol/L), and hypoproteinemia due to hypoalbuminemia (17 g/L; RR: 27 to 36 g/L). These findings were consistent with third space syndrome or free water excess attributed to hypotension secondary to congestive heart failure. Low total with normal ionized calcium was due to hypoalbuminemia, attributed to the combination of either free water excess, protein loss in the effusion, and/or decreased synthesis due to chronic inflammation. Complete blood (cell) count (CBC) revealed leukocytosis (17.8 × 109/L; RR: 5.1 to 11.0 × 109/L), neutrophilia (14.7 × 109/L; RR: 1.8 to 8.0 × 109/L) with regenerative left shift (bands of 0.5 × 109/L; RR: 0.0 × 109/L) and monocytosis (0.7 × 109/L; RR: 0.0 to 0.4 × 109/L), consistent with chronic active inflammation. Abdominal fluid analysis suggested non-septic neutrophilic inflammation. The horse was euthanized due to financial constraints and grave prognosis. Pericardial fluid was collected following euthanasia and analysis was consistent with suppurative inflammation. Culture of this sample was negative, contrary to the post-mortem sample (Table 3).
Table 3.
Bacterial culture results from 4 horses with septic fibrinous pericarditis referred to the Western College of Veterinary Medicine, University of Saskatchewan, between June and September 2017, following an environmental infestation with forest tent caterpillars.
| Sample | Bacteria isolated | |
|---|---|---|
| Case 1 | Necropsy: Liver, lung, pericardium | Enterococcus faecalis, Escherichia coli |
| Case 2 | On presentation: Pericardiocentesis | Negative |
| Necropsy: Liver, pericardium | Escherichia coli | |
| Necropsy: Pericardium | Actinobacillus suis | |
| Case 3 | On presentation: Pericardiocentesis | Actinobacillus suis, Actinobacillus equuli |
| Necropsy: pericardial and pleural fluid | Negative (post treatment with antimicrobials) | |
| Case 4 | On presentation: Pericardiocentesis following euthanasia | Negative |
| Necropsy: Pericardium | Actinobacillus suis, Escherichia coli |
Case 3
An 18-year-old Appaloosa mare was presented in early July for evaluation of lethargy and acute diarrhea. She had been with the current owner since she was 2 y old, not up-to-date on vaccinations but had been regularly dewormed (unknown protocol). She previously was presented in early June for lethargy and colic of undetermined cause. She had been managed symptomatically but had never fully recovered.
On presentation, the mare had lost body condition (BCS from 5 to 4/9) since the previous visit. On physical examination, right-sided congestive heart failure and pleural effusion were suspected (Tables 1 and 2). Thoracic ultrasound suggested fibrinous effusive pericarditis (Figure 1) and pleural effusion. Abdominal ultrasound revealed ascites and colonic edema suspected to be secondary to portal hypertension. Despite a poor to grave prognosis, the owner pursued further work-up and treatment. Blood analysis (venous blood gas, biochemistry, and CBC) results were comparable to Case 2. Cardiac troponin was 11 ng/L (RR: 0 to 60 ng/mL). Ultrasound-guided pericardiocentesis was performed before initiation of antimicrobial therapy and revealed septic suppurative inflammation (Table 3).
The mare received intravenous fluids including lactated Ringer’s solution (Baxter Corporation, Mississauga, Ontario) and 0.9% saline, started at maintenance rate [2 mL/kg body weight (BW) per hour] and adapted based on hydration status. Deficits were not addressed aggressively to avoid fluid overload, worsening edema, and third space losses. Broad spectrum antimicrobials included sodium penicillin G (Penicillin G for injection; Fresenius Kabi Canada, Toronto, Ontario), 22 000 IU/kg BW, IV, q6h, and gentamicin (Gentocin; Intervet Canada, Kirkland, Quebec), 6.6 mg/kg BW, IV, q24h. Therapeutic drug monitoring was submitted for gentamicin on day 4. Gentamicin concentrations were 18.1 μg/mL 1 h and 3.9 μg/mL 8 h after administration, resulting in a gentamicin concentration less than 2 μg/mL about 11 h after administration, which is desirable to avoid nephrotoxicity. The target peak of 8 to 10 times the minimum inhibitory concentration was also reached: 2 μg/mL was considered for the isolate of Actinobacillus (Table 3), reported susceptible to gentamicin. An anti-inflammatory drug (Flunixin injection; Norbrook Laboratories, Newry, Northern Ireland), 1.1 mg/kg BW, IV, q12h, was administered. The fever subsequently subsided and the respiratory rate normalized at the following physical examination 1 h later.
On day 2, a pericardial drain (24G French trocar catheter, length 40 cm; Argyle, Covidien, Mansfield, Massachusetts, USA) attached to a 3-way valve was placed under ultrasound guidance and concurrent electrocardiogram monitoring (Televet 100; Kruuse, Marlev, Denmark). Sixteen liters of fluid were drained. During the procedure, hypertonic saline (7.2% saline, 4 mL/kg BW) and colloids (6% hydroxyethyl starch, Voluven; Fresenius Kabi Canada, Toronto, Ontario), 5 mL/kg BW, were administered and the rate of IV polyionic fluid was increased (4 to 5 mL/kg BW per hour). Jugular distension and retrograde pulse resolved, and arterial pulse strength improved. Pericardial lavages (0.9% saline, 2 L, on day 2) and intrapericardial antimicrobial infusions (10 million IU penicillin G and 1 g gentamicin, twice on day 3) were performed. Stall side analysis (total solids, lactate) of the recovered pericardial fluid suggested response to treatment. Twelve hours after the second antimicrobial instillation, no fluid could be recovered. Either the drain was plugged by the accumulation of fibrin or there was not enough fluid accumulation to be drained; the drain was left in place for monitoring. No pericardial fluid had accumulated over 24 h, therefore, the drain was removed. Subsequent ultrasound examinations during hospitalization revealed no significant accumulation of pericardial fluid.
On day 3, the mare showed respiratory distress. Arterial blood gas analysis revealed hypoxemia (PO2: 61.0 mmHg) and hypercapnia (PCO2: 50.7 mmHg). Intranasal oxygen therapy was administered (5 L/min). A thoracic drain (24G French trocar catheter; Argyle, Covidien) connected to a Heimlich valve was placed under ultrasound guidance. The same IV fluid plan as for the placement of the pericardial drain was used. Fifty liters of fluid were drained from the thoracic cavity. Thoracic ultrasound revealed bilateral resolution of the effusion. Stall side analysis (total solids, lactate) of the fluid recovered suggested less severe inflammation compared with the pericardial fluid. The sample for cytology was lost during processing. Respiratory parameters returned to the normal range and oxygen therapy was discontinued. Fluid was drained for 3 d; when it stopped, the drain was clamped and removed 6 h after the absence of further fluid accumulation.
The mare returned to normal mentation and appetite. Fecal consistency normalized. Abdominal ultrasound revealed resolution of ascites and suggested resolution of colonic edema. Blood analysis suggested a response to treatment. Rebreathing examination was performed to confirm the absence of an underlying respiratory disease.
Due to financial constraints, the mare was discharged on day 8. She was switched to phenylbutazone (Phenylbutazone tablets 1000 mg; Dominion Veterinary Laboratories, Winnipeg, Manitoba), 2.2 mg/kg BW, PO, q12h, and trimethoprim/sulfamethoxazole (Apo-Sulfatrim-DS; Apotex, Toronto, Ontario), 30 mg/kg BW, PO, q12h, based on the susceptibility results. Vitamin E (3000E Vitamin E; Dominion Veterinary Laboratories), 6000 IU PO, q24h, was initiated for its potential myocardial antioxidant properties and pentoxifylline (Pentoxifylline SR; AA Pharma, Toronto, Ontario), 10 mg/kg BW, PO, q12h, was administered for its potential inhibitor effect of extracellular matrix proliferation which might decrease the incidence of constrictive pericarditis as previously reported (3). Even though these treatments have no proven efficacy, they were included for additional support in the context of the premature discharge of the horse from the hospital, at the owner’s request.
Follow-up included weekly physical examinations, CBC, and ultrasound examination. Eleven days after discharge, the mare had tachycardia, muffled heart sounds, jugular distension with retrograde pulses, coughing, thoracic and pericardial effusions, suggestive of a relapse of right-sided congestive heart failure. Euthanasia was elected. Cytology of pericardial fluid showed no evidence of inflammation and pleural fluid suggested mild septic inflammation. Based on the necropsy findings and the negative culture of the fluid samples (Table 3), the relapse was attributed to the accumulation of fibrin on the epicardium which had likely caused a constrictive cardiomyopathy.
Case 4
A 15-year-old Quarter Horse mare was referred in late September for evaluation of persistent lethargy of approximately 3 weeks’ duration. She had developed anorexia, mild colic, teeth grinding, and increased respiratory effort during the 3 d before referral. She had been owned for 7 y and had no previous health concerns other than suspected pituitary pars intermedia dysfunction. She was not up-to-date on vaccinations and had been last dewormed the week before presentation (unknown product).
On presentation, right-sided congestive heart failure was suspected (Tables 1 and 2). Ultrasonographic examination revealed tri-cavitary effusion with fibrinous pericarditis such as that found in Case 3 (Figure 1). Arterial blood gas analysis revealed hypoxemia (PO2: 72.9 mmHg) and hyponatremia. Other significant abnormalities on biochemistry panel included hypercreatinemia (146 μmol/L; RR: 52 to 126 μmol/L), hypoalbuminemia (21 g/L), and hyperglobulinemia (51 g/L; RR: 26 to 41 g/L). Prerenal azotemia was suspected due to decreased renal perfusion secondary to congestive heart failure. Hyperglobulinemia was attributed to antigenic stimulation from chronic inflammation. A CBC showed mild neutrophilia (9.1 × 109/L) with regenerative left shift (bands of 0.2 × 109/L) consistent with mild inflammation. The mare was euthanized based on a poor to grave prognosis.
Necropsy findings
Postmortem examination indicated severe fibrinous pericarditis and epicarditis with large amounts of pericardial effusion in all 4 cases (Figure 2). The pericarditis was classified as septic based on bacteriology results with evidence of bacteremia in Cases 1 and 2 (Table 3). There were variable amounts of thoracic and abdominal effusions in all affected horses. Histopathology revealed severe diffuse fibrinous pericarditis, granulation tissue formation, and variable fibroplasia in all cases. There were multifocal to coalescing foci of eosinophilic, lymphohistiocytic inflammation in the colon in 2 horses. Refractile yellowish brown foreign material was noted in the center of a few lesions in Case 2, which raised the suspicion of involvement of tent caterpillar setae (Figure 3).
Figure 2.
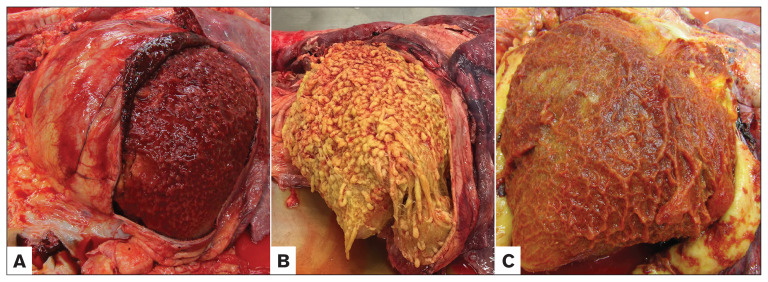
Post-mortem examination of the left side of the thorax from horses diagnosed with fibrinous pericarditis. Cranial is to the left. The pericardia have been opened and thick fibrinous material adhere to the epicardium. A — 3-year-old Quarter Horse. B — 8-year-old Quarter Horse. C — 18-year-old Appaloosa.
Figure 3.
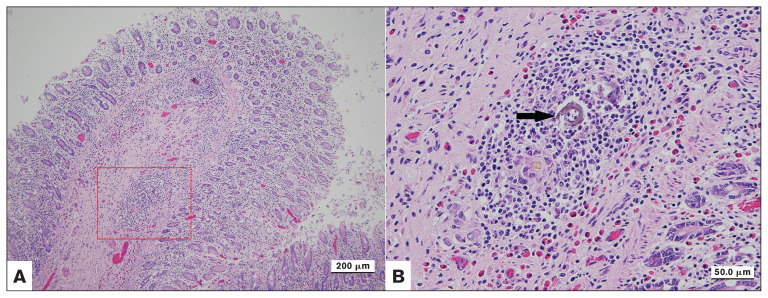
Eosinophilic lymphohistiocytic colitis with the cross section of a birefringent material from an 8-year-old Quarter Horse diagnosed with fibrinous pericarditis. B: is the zoom of the red square from picture A, arrow is the material.
Farm follow-up
None of the pen mates of Cases 2 and 3 had abnormalities on echocardiography. Two years later, all remaining horses were reported to be healthy.
Discussion
Congestive heart failure is a rare condition in horses and has a grave prognosis. The most common cause is valvular disease. Other causes include congenital disease, myocarditis, or pericarditis, which have a low prevalence (4). Three forms of pericarditis are reported including effusive, fibrinous, and constrictive (5). The effusive form lacks the presence of fibrin in the pericardial space and the constrictive form includes fibrosis of the pericardium (5), which was lacking in this case series. The present cases were all classified as septic fibrinous pericarditis. Pericarditis is most often idiopathic (5–9). Identified causes of pericarditis include immune-mediated conditions (5,6,10), which may be associated with viral disease (11), neoplasia (12–14), trauma including external trauma and foreign body (15,16), or vessel rupture (3). Bacterial infections (11,17–22) are reported and can be associated with mare reproductive loss syndrome (MRLS) (3,23–25).
The ingestion of eastern tent caterpillars (Malocosoma americanum) was associated with MRLS in Kentucky, Ohio, Indiana, Illinois, West Virginia, and Tennessee in 2001 and 2002 (23,26,27). The syndrome has been reproduced experimentally (27,28). The main bacteria recovered from tissues following abortion were non-hemolytic streptococci and actinobacilli which constitute part of the normal gastrointestinal flora of horses (28). Escherichia coli and Enterococcus spp. were also isolated in some cases, but seemed less frequent (28). Fibrinous pericarditis was associated with some cases of MRLS (24). Other conditions associated with MRLS include unilateral uveitis and rare cases of Actinobacillus meningoencephalitis (23). However, even if MRLS and pericarditis shared the same risk factor of caterpillar ingestion, a different pathophysiologic mechanism between the 2 conditions cannot be excluded (24).
It is still unclear if the ingestion of tent caterpillars induces an immune reaction due to an antigen or a toxin from the setae (3), or facilitates a direct hematologic spread of bacteria secondary to bacterial translocation from setae penetration through the colonic wall (29). A syndrome similar to MRLS, associated with the ingestion of processionary caterpillars (Ochrogaster lunifer), has been described in Australia and named equine amnionitis and fetal loss (EAFL) (30). This syndrome differs from MRLS by the period of abortion, the predominant bacteria isolated, and the absence of reported cases of pericarditis and uveitis. Therefore, it seems possible that pathogenicity can differ with the different species of caterpillars. An increased rate of abortions was not recognized in Saskatchewan during the forest tent caterpillar outbreak in 2017. This may have several explanations. First, the forest tent caterpillars may not be able to induce abortion in mares. Second, the breeding season period is delayed in Saskatchewan compared to the United States and Australia because of the long winters and short daylight hours. In MRLS, the window of abortions has been suggested to be between 40 to 120 d of pregnancy (27). Therefore, it is possible that the exposure of pregnant mares to caterpillars in Saskatchewan was at a different time of the gestational period than the potential window of abortion. Third, a breed factor cannot be excluded as most of the horses in Saskatchewan are Quarter Horse-related, whereas in Kentucky and Australia the breeds involved in the 2 syndromes were Thoroughbred-related. Finally, it is probable that abortions were simply not reported by owners and/or horses suffering from abortion, pericarditis, or uveitis were not referred to our hospital.
The clinical signs and clinicopathological abnormalities reported with pericarditis associated with MRLS are overall similar to the case series presented (3). The main difference is that all of the cases here were diagnosed with a septic process, whereas 67% of the cases of pericarditis reported in 2001 had sterile pericardial effusion (3). When septic effusions were present, Actinobacillus equuli, Streptococcus spp., Pasteurella multocida, Staphylococcus aureus, Pseudomonas spp., or Acinetobacter spp. were isolated (3). In another report, 40% of the pericarditis associated with caterpillar ingestion yielded predominantly Actinobacillus spp., but also E. coli and Enterococcus faecalis (25). In addition, Cases 2 and 4 highlighted the fact that some pericarditis can be falsely classified as idiopathic when solely pericardiocentesis is used for analysis. This previously lead some authors to recommend more invasive diagnostic tests such as pericardial or myocardial biopsy to decrease the number of false diagnoses of idiopathic cases (31). In human medicine, recommendations for diagnostic tests include physical examination, blood analysis, imaging and, electrocardiography, followed by analysis of fluid from pericardiocentesis. Biopsy should only be considered in suspected cases of tuberculosis and neoplasia when previous tests did not reach a final diagnosis; it has a lower recommendation status then the first tests listed (32).
Outbreaks of forest tent caterpillars are unpredictable and lack periodicity in Saskatchewan compared to the outbreaks in eastern Canada (33). Records from the last 20 y at the WCVM were reviewed by the authors to investigate the frequency of pericarditis diagnosed at our hospital. Horses were included if they presented signs of heart failure or if they were diagnosed with pericarditis on necropsy. From 1996 to 2018, 35 horses were presented with signs of heart failure. Final diagnoses were available for only 17 horses, and only the 4 horses presented in this case series had a final diagnosis of fibrinous pericarditis. There is little information available about the severity of the outbreak of caterpillars in 2017 compared with previous outbreaks to confirm with certainty that the infestation was more severe than usual. Based on Internet and social media searches, the years 2016 and 2017 seemed to have a particular abundance of caterpillars compared to previous years (1). However, no further conclusion can be drawn about an association between the 2017 outbreak of forest tent caterpillars and this case series of pericarditis. It is also very likely that even if a real cause-effect relationship existed, not all cases were referred to the WCVM. Based on the economics of the horse industry in Saskatchewan, the severity of the clinical signs of heart failure and the poor prognosis, it is likely that most horses, once diagnosed, are euthanized on farm without a necropsy performed.
The main support for a possible association between the 2017 forest tent caterpillar outbreak in Saskatchewan and the occurrence of several cases of fibrinous pericarditis includes the exposure to forest tent caterpillars, the number of cases of this rare condition presented only in 2017 (since 1996), the homogeneity of the clinical signs, and pathological findings. The presence of granulomatous colitis was reported in experimental models of mares fed eastern tent caterpillars (2) and processionary caterpillars (34). In these reports, setae were also found on histology of the intestinal walls. They had a similar appearance to the birefringent material described in the histology of Case 2. However, this foreign body could not be further analyzed, and other types of foreign bodies could not be ruled out.
In previous reports, pericarditis was successfully treated in 67% to 70% of cases (9). Only the owner of the horse in Case 3 elected to pursue treatment. The mare relapsed following initial response to treatment, but post-mortem examination suggested that the initial treatment had successfully resolved the pericardial infection. The relapse was attributed to the mechanical constrictive effect from the fibrin accumulated on the epicardium. Further treatment options could have included fibrinolytic agents (streptokinase, tissue plasminogen activator), corticosteroids, heparin, and hyaluronic acid (3). These were not initiated due to the cost of such treatments.
To the authors’ knowledge, this is the first report of a suspected association between an outbreak of forest tent caterpillars and equine fibrinous pericarditis in western Canada. Based on the information available, it seems prudent to avoid, as much as possible, the exposure and accidental ingestion of caterpillars by horses. Interestingly, in Kentucky, co-grazing with cattle has been reported as being protective (24). Some pesticides are also effective in controlling the infestation of eastern tent caterpillars (35). This report can serve as a starting point for prospective epidemiologic research on pathology caused by forest tent caterpillars in horses or for a clinical trial.
Acknowledgments
Case 3 was presented by Gillian Davies (WCVM class of 2020), supervised by Drs. Ronan Chapuis and Julia Montgomery, as part of a student case report competition on a French veterinary website. Ms. Davies received a student report award (www.vetofocus.com). CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.“They just keep coming”: Tent caterpillar invasion coats Sask. home in insects — and feces. CBC News (updated May 31, 2017) [Last accessed April 9, 2020]. Available from: https://www.cbc.ca/news/canada/saskatchewan/tent-caterpillars-rm-dundurn-1.4139552.
- 2.McDowell KJ, Webb BA, Williams NM, et al. Invited review: The role of caterpillars in mare reproductive loss syndrome: A model for environmental causes of abortion. J Anim Sci. 2010;88:1379–1387. doi: 10.2527/jas.2009-2584. [DOI] [PubMed] [Google Scholar]
- 3.Slovis N. Pericarditis: A clinical perspective during an epidemic of fibrinous pericarditis in central Kentucky. Equine Vet Educ. 2011;23:69–72. [Google Scholar]
- 4.Davis JL, Gardner SY, Schwabenton B, Breuhaus BA. Congestive heart failure in horses: 14 cases (1984–2001) J Am Vet Med Assoc. 2002;220:1512–1515. doi: 10.2460/javma.2002.220.1512. [DOI] [PubMed] [Google Scholar]
- 5.Worth LT, Reef VB. Pericarditis in horses: 18 cases (1986–1995) J Am Vet Med Assoc. 1998;212:248–253. [PubMed] [Google Scholar]
- 6.Freestone JF, Thomas WP, Carlson GP, Brumbaugh GW. Idiopathic effusive pericarditis with tamponade in the horse. Equine Vet J. 1987;19:38–42. doi: 10.1111/j.2042-3306.1987.tb02576.x. [DOI] [PubMed] [Google Scholar]
- 7.Malalana F, Bardell D, McKane S. Idiopathic aseptic pericardial effusion with cardiac tamponade in a horse. Equine Vet J. 2011;23:64–68. [Google Scholar]
- 8.Dill SG, Simoncini DC, Bolton GR, et al. Fibrinous pericarditis in the horse. J Am Vet Med Assoc. 1982;180:266–271. [PubMed] [Google Scholar]
- 9.Reimer J. Management of equine pericarditis. Equine Vet Educ. 2013;25:334–338. [Google Scholar]
- 10.Robinson JA, Marr CM, Reef VB, Sweeney RW. Idiopathic, aseptic, effusive, fibrinous, nonconstrictive pericarditis with tamponade in a standardbred filly. J Am Vet Med Assoc. 1992;201:1593–1598. [PubMed] [Google Scholar]
- 11.Jesty SA, Reef VB. Septicemia and cardiovascular infections in horses. Vet Clin North Am Equine Pract. 2006;22:481–495. doi: 10.1016/j.cveq.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Carnine BL, Schneider G, Cook JE, Leipold HW. Pericardial mesothelioma in a horse. Vet Pathol. 1977;14:513–515. doi: 10.1177/030098587701400512. [DOI] [PubMed] [Google Scholar]
- 13.Stoica G, Cohen N, Mendes O, Kim HT. Use of immunohistochemical marker calretinin in the diagnosis of a diffuse malignant metastatic mesothelioma in an equine. J Vet Diagn Invest. 2004;16:240–243. doi: 10.1177/104063870401600313. [DOI] [PubMed] [Google Scholar]
- 14.Hargreaves L, Gosling L, Dixon JJ. Pericardial effusion and congestive heart failure in a horse with multicentric lymphoma. Vet Rec Case Rep. 2018;6:e000631. [Google Scholar]
- 15.Bertone JJ, Dill SG. Traumatic gastropericarditis in a horse. J Am Vet Med Assoc. 1985;187:742–743. [PubMed] [Google Scholar]
- 16.Voros K, Felkai C, Szilagyi Z, Papp A. Two-dimensional echocardiographically guided pericardiocentesis in a horse with traumatic pericarditis. J Am Vet Med Assoc. 1991;198:1953–1956. [PubMed] [Google Scholar]
- 17.Benson CE, Sweeney CR. Isolation of Streptococcus pneumoniae type 3 from equine species. J Clin Microbiol. 1984;20:1028–1030. doi: 10.1128/jcm.20.6.1028-1030.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkins SL, Magdesian KG, Thomas WP, Spier SJ. Pericarditis and pleuritis caused by Corynebacterium pseudotuberculosis in a horse. J Am Vet Med Assoc. 2004;224:1133–1138. doi: 10.2460/javma.2004.224.1133. [DOI] [PubMed] [Google Scholar]
- 19.May KA, Cheramie HS, Howard RD, et al. Purulent pericarditis as a sequela to clostridial myositis in a horse. Equine Vet J. 2002;34:636–640. doi: 10.2746/042516402776180278. [DOI] [PubMed] [Google Scholar]
- 20.Morley PS, Chirino-Trejo M, Petrie L, Krupka L, Schwab M. Pericarditis and pleuritis caused by Mycoplasma felis in a horse. Equine Vet J. 1996;28:237–240. doi: 10.1111/j.2042-3306.1996.tb03779.x. [DOI] [PubMed] [Google Scholar]
- 21.Alcott C, Howard J, Wong D, Haynes J. Fibrinous pericarditis and cardiac tamponade in a 3-week-old pony foal. Equine Vet Educ. 2013;25:328–333. [Google Scholar]
- 22.Armstrong SK, Raidal SL, Hughes KJ. Fibrinous pericarditis and pericardial effusion in three neonatal foals. Aust Vet J. 2014;92:392–399. doi: 10.1111/avj.12238. [DOI] [PubMed] [Google Scholar]
- 23.Sebastian MM, Bernard WV, Riddle TW, Latimer CR, Fitzgerald TD, Harrison LR. REVIEW paper: Mare reproductive loss syndrome. Vet Pathol. 2008;45:710–722. doi: 10.1354/vp.45-5-710. [DOI] [PubMed] [Google Scholar]
- 24.Seahorn JL, Slovis NM, Reimer JM, Carey VJ, Donahue JG, Cohen ND. Case-control study of factors associated with fibrinous pericarditis among horses in central Kentucky during spring 2001. J Am Vet Med Assoc. 2003;223:832–838. doi: 10.2460/javma.2003.223.832. [DOI] [PubMed] [Google Scholar]
- 25.Bolin DC, Donahue JM, Vickers ML, et al. Microbiologic and pathologic findings in an epidemic of equine pericarditis. J Vet Diagn Invest. 2005;17:38–44. doi: 10.1177/104063870501700108. [DOI] [PubMed] [Google Scholar]
- 26.Dwyer RM, Garber LP, Traub-Dargatz JL, et al. Case-control study of factors associated with excessive proportions of early fetal losses associated with mare reproductive loss syndrome in central Kentucky during 2001. J Am Vet Med Assoc. 2003;222:613–619. doi: 10.2460/javma.2003.222.613. [DOI] [PubMed] [Google Scholar]
- 27.Bernard WV, LeBlanc MM, Webb BA, Stromberg AJ. Evaluation of early fetal loss induced by gavage with eastern tent caterpillars in pregnant mares. J Am Vet Med Assoc. 2004;225:717–721. doi: 10.2460/javma.2004.225.717. [DOI] [PubMed] [Google Scholar]
- 28.Webb BA, Barney WE, Dahlman DL, et al. Eastern tent caterpillars (Malacosoma americanum) cause mare reproductive loss syndrome. J Insect Physiol. 2004;50:185–193. doi: 10.1016/j.jinsphys.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Donahue JM, Sells SF, Bolin DC. Classification of Actinobacillus spp. isolates from horses involved in mare reproductive loss syndrome. Am J Vet Res. 2006;67:1426–1432. doi: 10.2460/ajvr.67.8.1426. [DOI] [PubMed] [Google Scholar]
- 30.Cawdell-Smith AJ, Todhunter KH, Perkins NR, Bryden WL. Exposure of mares to processionary caterpillars (Ochrogaster lunifer) in early pregnancy: An additional dimension to equine amnionitis and fetal loss. Equine Vet J. 2013;45:755–760. doi: 10.1111/evj.12044. [DOI] [PubMed] [Google Scholar]
- 31.Sage A. Fever: Endocarditis and pericarditis. In: Marr C, Bowen M, editors. Cardiology of the Horse. 2nd ed. Philadelphia, Pennsylvania: Saunders; 2011. pp. 217–225. [Google Scholar]
- 32.Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooke BJ, Lorenzetti F. The dynamics of forest tent caterpillar outbreaks in Québec, Canada. Forest Ecol Manag. 2006;226:110–121. [Google Scholar]
- 34.Todhunter KH, Cawdell-Smith AJ, Bryden WL, Perkins NR, Begg AP. Processionary caterpillar setae and equine fetal loss: 1. Histopathology of experimentally exposed pregnant mares. Vet Pathol. 2014;51:1117–1130. doi: 10.1177/0300985813516638. [DOI] [PubMed] [Google Scholar]
- 35.Potter DA, Foss L, Baumler RE, Held DW. Managing Eastern tent caterpillars Malacosoma americanum (F) on horse farms to reduce risk of mare reproductive loss syndrome. Pest Manag Sci. 2005;61:3–15. doi: 10.1002/ps.958. [DOI] [PubMed] [Google Scholar]