Abstract
Background
miRNAs have been widely used in cancer treatment. Our study was designed to explore the effects of miR-325-3p in bladder cancer cells.
Material/Methods
Levels ofd miR-325-3p and MT3 in bladder cancer tissues and cells were assessed by quantitative real-time polymerase chain reaction (qRT-PCR). miR-325-3p mimics were transfected into bladder cancer T24 cells, and cell migration and invasion rates and cell proliferation were assessed by transwell assay and Cell Counting Kit-8 (CCK-8). The target mRNA for miR-325-3p was predicted by Targetscan7.2 and confirmed by dual-luciferase reporter assay. More experiments were performed to confirm the effects of miR-325-3p and MT3 in T24 cells. Additionally, the levels of TIMP-2, MMP9, and E-cadherin were assessed by Western blotting to identify the effects of miR-325-3p and MT3 on epithelial-mesenchymal transition (EMT).
Results
miR-325-3p expression was reduced and MT3 was increased in bladder cancer tissues and bladder cancer cells. miR-325-3p mimics suppressed cell proliferation ability and invasion and migration rates of T24 cells. Moreover, miR-325-3p was confirmed to target MT3. Further experiments showed that the effects of increased cell proliferation, invasion, migration, and EMT promoted by MT3 overexpression were abolished by miR-325-3p mimics, proving that miR-325-3p is a tumor suppressor through targeting MT3 in bladder cancer cells.
Conclusions
Downregulation of miR-325-3p in bladder cancer regulates cell proliferation, migration, invasion, and EMT by targeting MT3. Furthermore, miR-325-3p is a potential therapeutic target in treating bladder cancer.
MeSH Keywords: Cell Proliferation, Metallothionein, MicroRNAs, Neoplasm Metastasis, Urinary Bladder Neoplasms
Background
Bladder cancer is common worldwide, with men having a higher incidence rate than women [1] and women having more advanced stage and less favorable outcomes than men [2]. In addition to cytotoxic cisplatin-based chemotherapy, FGFR3 inhibitor, immune checkpoint inhibitor [3,4], neoadjuvant chemotherapy (NAC), and molecular-targeted therapy have been developed as promising strategies for treating bladder cancer, but disease recurrence, progression, and mortality after treatment still remains great challenges to be solved. Therefore, it is important to explore the molecular mechanism of bladder cancer.
MicroRNAs (miRNAs) are short non-coding RNAs that degrade or reduce the translation of target mRNAs. Dysregulation of miRNAs has been observed in most cancers, and is related to tumorigenesis [5], proliferation [6], metastasis [7], chemoresistance [8], and stemness [9]. Recently, miRNA signature in the early stages of gastric tumorigenesis has been observed to restore miR143-3p expression or inhibit its target through BRD2, which might be applied for treatment of gastric cancer [10]. Additionally, miRNAs are potential predictors for urologic cancer survival [11]. In lung cancer, miR-325-3p has been found to be downregulated, and it regulates cell proliferation and invasion via targeting HMGB1 [12], while in hepatocellular carcinoma, miR-325-3p suppresses cell growth by downregulating aquaporin 5 [13]. miR-325 may be a potential biomarker of bladder cancer [14]. The possible molecular mechanism of miRNA-325 in bladder cancer still needs to be further researched.
Metallothioneins (MTs) are small cysteine-rich proteins that regulate homeostasis of metal and prevent cells from heavy metal-induced cytotoxicity. MTs have been found to be downregulated in multiple cancers, and are involved in tumor angiogenesis, tumor differentiation, immunomodulation, drug resistance, tumor growth, and metastasis [15]. MTs have 4 main isoforms (MT1, MT2, MT3, and MT4) in humans, and MT3 is one of the 4 main isoforms. MT3 is downregulated in breast cancer cell lines [16], but is enhanced in primary prostate cancer cells [17]. Additionally, MT3 is an oncogene, and promotes the development of bladder carcinoma [18]. Nonetheless, the function and mechanism of MT3 in bladder cancer and whether miR-325 targets MT3 in bladder cancer should be further researched.
This study was designed to investigate the roles of miR-325-3p and MT3 in bladder cancer, hoping to find a potential therapeutic target based on miRNA.
Material and Methods
Patients and tissue sample
From June 2017 to July 2018, 30 bladder cancer tissues and adjacent normal tissues were collected from bladder cancer patients after surgical operations. The current research was approved by the Ethics Committee of Beijing Luhe Hospital Affiliated to Beijing Capital Medical University. All patients signed informed consent for sample collection. All histological and pathologic diagnoses were confirmed by 3 pathologists. All specimens were stored at −80°C.
Cell culture
Human normal ureter epithelial cell SV-HUC-1 (CRL-9520), bladder cancer cells lines [SW780 (CRL-2169), and HT1376 (CRL-1472)] were obtained from the American Type Culture Collection (USA). Another 3 bladder cancer cells lines (T24, J82 and UMUC3) were from the cell bank of the Chinese Academy Sciences Typical Culture Preservation Committee (China). SV-HUC-1 cells were cultured in F-12K medium; SW780 cells were cultured in Leibovitz’s L-15 (11415056, Gibco, ThermoFisher, USA); HT1376 and J82 cells were cultured in MEM medium (11095114, Gibco, ThermoFisher, USA); T24 cells were cultured in McCoy’s 5a medium (16600082, Gibco, ThermoFisher, USA); and UMUC3 cells were cultured in MEM-a medium (32561037, Gibco, ThermoFisher, USA). All media were supplemented with 10% FBS (16140071, Gibco, ThermoFisher, USA), and 1% penicillin/streptomycin (15140163, Gibco, ThermoFisher, USA). All cells were cultured in 5% CO2 at 37°C.
Cell transfection
miR-325-3p mimics (5′-UACAGGUUAGAUUAUGUACU-3′), control mimics, and scramble negative control were purchased from RiboBio Co. Opti-MEM (11058021, Invitrogen, USA) containing Lipofectamine® 2000 (11668019, Invitrogen, USA) was used to transfect miR-325-3p mimic and control mimics into T24 cells at room temperature according to the instructions of the manufacturer. Cells were incubated at 37°C for 24 h and collected for further experiments.
Full-length cDNA sequence of MT3 was amplified and inserted into the pcDNA 3.1 plasmid (V79020, Invitrogen, ThermoFisher, USA). The pcDNA 3.1-MT3 was then transfected into T24 cells for overexpressing MT3. Cells were incubated at 37°C for 24 h and collected for further experiments.
Qualitative real-time PCR analysis
Tissue specimens and cells were treated by Trizol reagent (15596018, Invitrogen, ThermoFisher, USA) on ice. NanoDrop 8000 (ND-8000-GL, Thermo Scientific, USA) was used for determining the concentration of RNA.
For the quantification of MT3, TIMP-2, MMP9, and E-cadherin, reverse-transcription was performed using the PrimeScript™ II First Strand cDNA Synthesis Kit (6210B, Takara, Japan). qRT-PCR was performed with SYBR® Green PCR Master Mix (4312704, ABI, USA). GAPDH served as a reference gene. For quantification of miR-325-3p, reverse-transcription was performed using the One-step miRNA RT kit (D1801, HaiGene, China). qRT-PCR was performed with the SYBR Green qPCR kit (C10211-1, RiboBio, China). U6 snRNA served as a reference gene. Reaction conditions for all qRT-PCR were: 95°C (5 min), 40 cycles with 95°C (15 s), 60°C (30 s), and 70°C (10 s). The Bio-Rad CFX 96 Touch Real-Time PCR Detection System (1855196, Bio-Rad, China) and the comparative threshold cycle method 2−ΔΔCT were used to calculate the relative expressions. All reactions were performed in triplicate. All primers for qRT-PCR are shown in Table 1.
Table 1.
Primers used for qRT-PCR.
| Gene | Forward | Reverse |
|---|---|---|
| miR-325-3p [22] | ATATAGTGCTTGGTTCCTAGTAGGTGC | CCAGAGCCTAGCACAGTGCT |
| MT3 | ATGGACCCTGAGACCTGCCC | TCACTGGCAGCAGCTGCACT |
| TIMP-2 | AAGCGGTCAGTGAGAAGGAAG | GGGGCCGTGTAGATAAACTCTAT |
| MMP9 | TGTACCGCTATGGTTACACTCG | GGCAGGGACAGTTGCTTCT |
| E-cadherin | CGAGAGCTACACGTTCACGG | GGGTGTCGAGGGAAAAATAGG |
| U6 | GTCCCTTCGGGGACATCC | AAAATTTTGGACCATTTCTCGATTT |
| GAPDH | GCTCT CTGCTCCTCCTGTTC | TAACTGGTTGAGCACAGGGTAC |
Cell Counting Kit-8 (CCK-8) assay
After the transfection, the cells (5000 cells/well) were grown in 96-well plates. After incubation for 24, 48, or 72 h, 10 μL CCK-8 (70-CCK801, MultiSciences, China) was dropped into the well and bathed at 37°C for 4 h. Next, cell viability was measured at a wave length of 450 nm. All experiments were conducted in triplicate.
Transwell assays
For detecting cell migration ability, a transwell chamber (3422, Corning, USA) was inserted into the 24-well plates. Then, cells in serum-free medium were added into the upper chamber. Serum (20%) containing medium was placed into the lower chamber. After being incubated for 48 h at 37°C, cells migrating through the filters were counted under a microscope (TS100, Nikon, Japan). For invasion assay, the pores in the membrane were blocked by matrigel (354248, Corning, USA), and similar operations were conducted as described above. Each experiment was independently repeated in triplicate.
Dual-luciferase reporter assay
PMIR-reporter luciferase vector was used for luciferase assays. Wild-type and mut-type MT3 3′UTR were linked to pMIR vector, and then transfected into T24 cells. Control mimics, scramble control mimics, or miR-325-3p mimic (15 nM) were transfected into the T24 cells. After incubation for 24 h, a dual-luciferase reporter assay system (N1610, Promega, USA) with a GloMax® Discover System (GM3000, Promega, USA) was used to determine the luciferase activity.
Western blotting
Tissues or cells were treated by RIPA lysis buffer (89901, Thermo Scientific, USA) containing PMSF protease inhibitor (36978, Thermo Scientific, USA), for protein extraction. Next, the total proteins were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (LC2002, Invitrogen, ThermoFisher, USA), which were blocked by 5% of non-fat milk (PA201-01, BioMed, China) for 10 min at room temperature. Membranes were then exposed to primary antibodies (anti-MT3 antibody (1: 1000, SAB1402849, Sigma, USA); Anti-TIMP-2 antibody (1: 2000, ab180630, Abcam, UK), Anti-MMP9 antibody (1: 2000, ab38898, Abcam, UK) and Anti-E-cadherin antibody (1: 2000, ab15148, Abcam, UK), or Anti-GAPDH antibody (ab181602, 1: 2000, Abcam, UK) at 4°C for 8 h. The membranes were then incubated with anti-Mouse IgG (1: 2000, 70-GAM007, MultiSciences, China) with MT3 and anti-rabbit IgG antibody (1: 2000, 7074, Cell Signaling Technology, USA) for TIMP-2, MMP9, MMP9, and GAPDH at 4°C overnight. After each incubation, the membrane was washed by 1×TBST (50 mM Tris, 150 mM NaCl and 2% Tween-20; pH 7.5). Finally, SignalFire™ ECL reagent (6883, Cell Signaling Technology, USA) was applied to collect the signals. GADPH served as an internal control.
Statistical analysis
SPSS v.19.0 software (IBM, Armonk, NY, USA) was used for data analyses and GraphPad Prism 5.02 (La Jolla, CA, USA) was used for graphing. The data are expressed as the mean±SD, and analyzed by t test or Tukey’s post hoc test after ANOVA. P<0.05 indicates a significant difference.
Results
miR-325-3p was reduced in bladder cancer tissue and cells
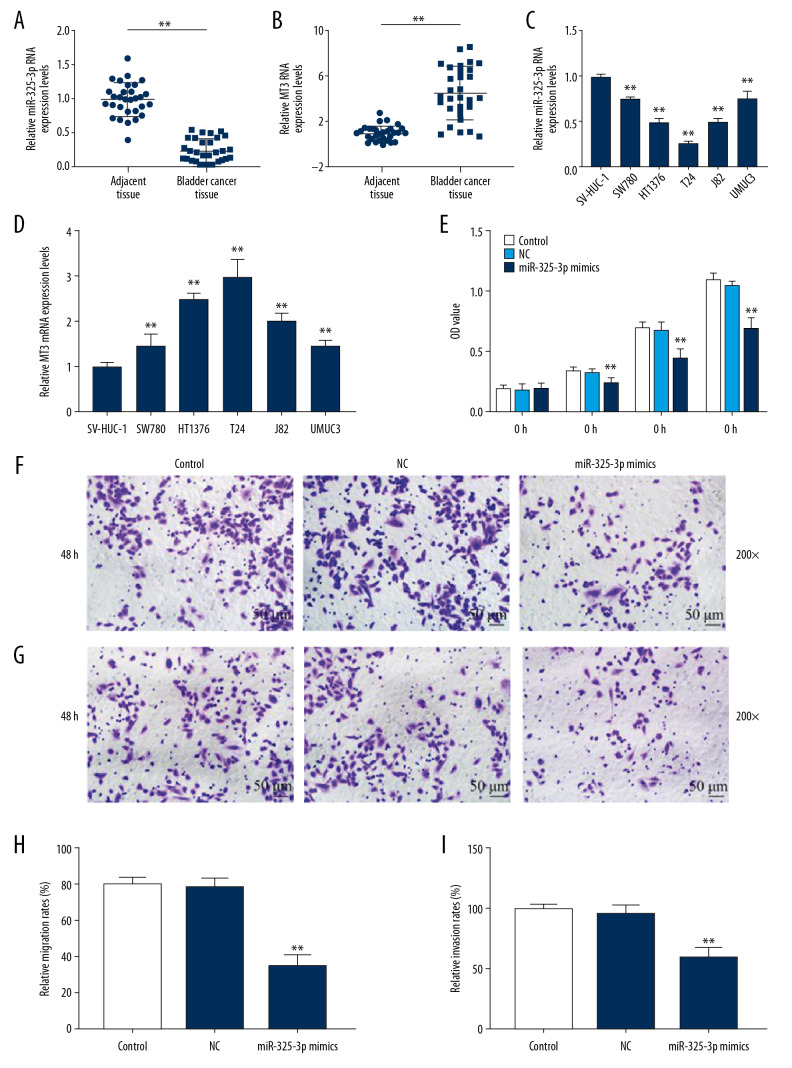
To assess the effects of miR-325-3p in bladder cancer, we first measured the level of miR-325-3p in bladder cancer tissues and cells lines. The qRT-PCR results showed that miR-325-3p was downregulated (P<0.001, Figure 1A, 1C) and MT3 was upregulated (P<0.001, Figure 1B, 1D) in bladder cancer tissues and bladder cancer cell lines, compared with tumor-adjacent normal bladder tissues and normal ureter epithelial cell SV-HUC-1, respectively. As miR-325-3p expression in T24 cells was the lowest among all detected bladder cancer cells, T24 cells were used in further research.
Figure 1.
The expression pattern and effects of miR-325-3p on bladder cancer tissues and cells. (A) The expression levels of miR-325-3p in tumor-adjacent normal bladder tissues (n=30) and bladder cancer tissues (n=30) were measured by qRT-PCR. * Vs. adjacent tissue. * P<0.05; ** P<0.001. (B) The expression levels of MT3 in tumor-adjacent normal bladder tissues (n=30) and bladder cancer tissues (n=30) were measured by qRT-PCR. * Vs. adjacent tissue. * P<0.05; ** P<0.001. (C) The expression levels of miR-325-3p in different bladder cell lines (SV-HUC-1, SW780, HT1376, T24, J82 and UMUC3 cells) were measured by qRT-PCR. * Vs. SV-HUC-1. * P<0.05; ** P<0.001. (D) The expression levels of MT3 in different bladder cell lines (SV-HUC-1, SW780, HT1376, T24, J82 and UMUC3 cells) were measured by qRT-PCR. * Vs. SV-HUC-1. * P<0.05; ** P<0.001. (E) The proliferation capacity of T24 cells was measured by CCK-8 assays after interference with control, NC or miR-325-3p mimics at 0 h, 24 h, 48 h and 72 h. (F, H) The cell migration of T24 cells was evaluated by transwell assay at 200x. The migration rate of T24 cells was measured in the right chart. (G, I) The cell invasion of T24 cells was evaluated by transwell assay at 200x. The invasion rate of T24 cells was shown in the right chart. * Vs. NC. * P<0.05; ** P<0.001. Control – non-specific target control; NC – scrambled negative control.
miR-325-3p mimics suppressed cell proliferation, migration, and invasion in T24 cells
After the transfection of miR-325-3p mimics into T24 cells, CCK-8 assay showed that the proliferation rate of T24 cells was reduced by miR-325-3p mimics (P<0.001, Figure 1E). Transwell assays indicated that the cell migration and invasion were also reduced by miR-325-3p mimics (P<0.001, Figure 1F–1I).
miR-325-3p regulated the expression level of MT3 via targeting 3′UTR of MT3
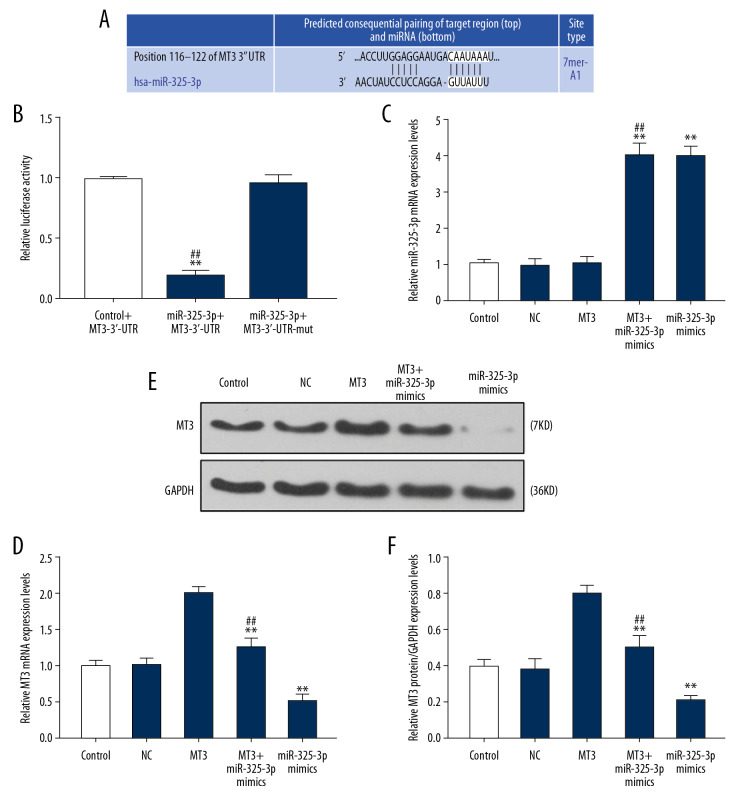
TargetScan7.2 predicted that 3′UTR (site 116–122) of MT3 mRNA was possibly the target of miR-325-3p (Figure 2A), and the prediction was verified by dual-luciferase reporter assay, as the luciferase activity of T24 cells transfected with MT3-3′-UTR and miR-325-3p mimics was significantly reduced (P<0.001), while there was little change in T24 cells co-transfected with miR-325-3p mimics and MT3-3′-UTR-mut (Figure 2B). In addition, qRT-PCR determined that miR-325-3p mimics downregulated the level of MT3 (P<0.001, Figure 2D), while overexpression of MT3 did not influence miR-325-3p expression (P<0.001, Figure 2C). Western blot analysis demonstrated that miR-325-3p mimics also downregulated the expression level of MT3 (P<0.001, Figure 2E, 2F).
Figure 2.
miR-325-3p regulated the expression level of MT3 via targeting 3′UTR of MT3 mRNA in T24 cells. (A) The potential target site for miR-325-3p in the 3′UTR of the MT3 mRNA predicted by Targetscan7.2. (B) Double-luciferase reporter assays were used to validate miR-325-3p binding at the 3′UTR of MT3 in T24 cells. * Vs. Control+MT3-3′-UTR; # Vs. miR-325-3p+MT3-3′-UTR-mut. * P<0.05; ** P<0.001. (C) The relative mRNA expression level of miR-325-3p was measured by qPCR in T24 cells. (D, E) QRT-PCR analysis (D) and Western blot (E, F) showed the expression level of MT3 in T24 cells. * Vs. NC; # vs. MT3. * P<0.05; ** P<0.001. Control – non-specific target control; NC – scrambled negative control; MT3+ miR-325-3p mimics – co-transfection with MT3 overexpression plasmid and miR-325-3p mimics.
miR-325-3p regulated cell proliferation, migration, invasion, and EMT through targeting MT3
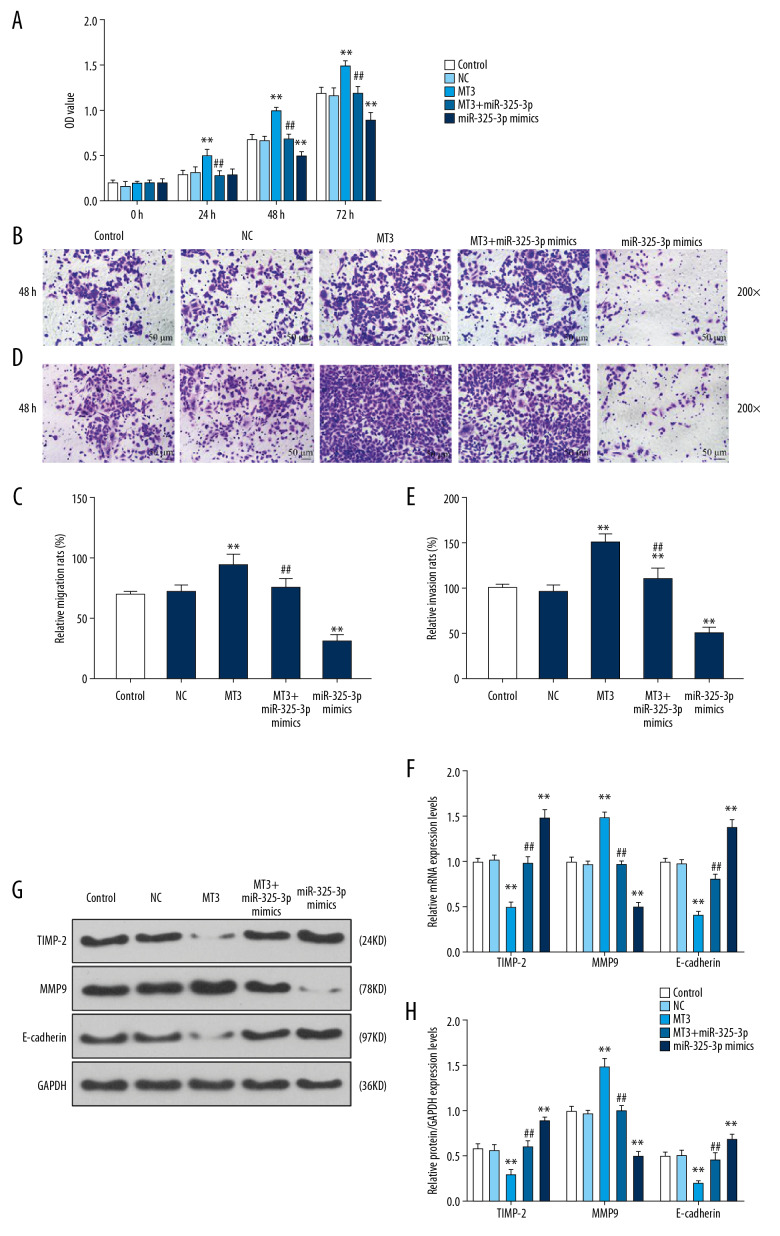
To further explore the effects of miR-325-3p on bladder cancer cells through targeting MT3, we measured the cell proliferation, cell migration, and invasion of T24 cells transfected with miR-325-3p mimics or MT3 overexpression plasmid. The results showed that overexpression of MT3 increased the cell proliferation rate, while miR-325-3p mimics reversed the effect of MT3 overexpression (P<0.001, Figure 3A). The increased migration and invasion of T24 cells with MT3 overexpression were also rescued by miR-325-3p mimics (all P<0.001, Figure 3B–3E).
Figure 3.
miR-325-3p regulated proliferation, migration, invasion, and EMT via targeting MT3. (A) The proliferation capacity of T24 cells transfected with control, NC, miR-325-3p mimics or MT3 plasmid at 0 h, 24 h, 48 h, and 72 h was measured by CCK-8 assays. (B, C) The cell migration of T24 cells was evaluated by transwell assay at 200×. The migration rate of T24 cells was measured in the right chart. (D, E) The cell invasion of T24 cells was evaluated by transwell assay at 200×. The invasion rate of T24 cells was shown in the right chart. (F-H) QRT-PCR analysis (F) and Western blot (G, H) showed the expression levels of TIMP-2, MMP9 and E-cadherin. * Vs. NC; # vs. MT3. * P<0.05; ** P<0.001. Control – non-specific target control; NC – scrambled negative control; MT3+ miR-325-3p mimics – co-transfection with MT3 overexpression plasmid and miR-325-3p mimics.
To investigate the effects of miR-325-3p mimics on EMT, TIMP-2, MMP9, and E-cadherin were measured to reflect the EMT state in T24 cells. The data demonstrated that overexpression of MT3 reduced the expressions of TIMP-2 and E-cadherin (both P<0.001), and increased the expression of MMP9 (P<0.001), which, however, were reversed by miR-325-3p mimics (P<0.001, Figure 3F–3H).
Discussion
miRNAs are key modulators in cancers. miRNAs are frequently dysregulated in bladder cancers, and many of them are functionally involved in the pathogenesis of cancer. In this study, we found that miR-325-3p is a tumor suppressor to bladder cancer through inhibiting cell proliferation, migration, and invasion via targeting MT3. We are the first to demonstrate the relationship between miR-325-3p and MT3 in bladder cancer.
Recently, miRNAs have become potential therapeutic tools in treatment of cancers, as cancer-related miRNAs function as oncogenes or suppressors [19,20]. In non-small cell lung cancer, miRNAs are used in anti-metastatic therapy [21]. The cell-cycle-targeting miRNAs are used for inhibiting excessive tumor growth [22]. Thus, we were interested in the potential inhibitive function of miR-325-3p mimics on bladder cancer. In the current study, we found that miR-325-3p was significantly reduced in bladder cancer tissues and cells, suggesting that miR-325-3p was potentially related to the process of bladder cancer. Furthermore, we found that miR-325-3p overexpressed by its mimics reduced the cell proliferation, migration, and invasion rate, but function of miR-325-3p overexpression in bladder cancer should be further verified in vivo.
Previous studies showed that miR-325-3p has several targets genes; for example, miR-325-3p negatively regulates EGFR [23]. A study also confirmed a direct interaction between miR-325-3p and AANAT mRNA in hypoxic-ischemic brain damage rats [24]. In our study, we found that MT3 could be targeted by miR-325-3p in bladder cancer cells. We hypothesized that miR-325-3p, like other miRNAs, has multiple target genes and plays complicated roles in the development of cancer. MT3 is one of the 4 main isoforms in the metallothioneins (MTs) family, and promotes the invasion of triple-negative breast cancer through upregulating metalloproteinase [25]. MT3 is also induced by hypoxia to enhance tumorigenesis and cell invasion ability of bladder carcinoma [18]. However, a study suggested that MT3 is a tumor suppressor and is frequently downregulated in acute myeloid leukemia [26]. Thus, the function of MT3 in cancer remains unclear. We found that MT3 was the target for miR-325-3p. Further experiments demonstrated that MT3 is more likely an oncogene in bladder cancer, and overexpression of MT3 increased the growth and metastasis of bladder cancer cells, which was consistent with most previous studies. We confirmed that MT3 is the functional target for miR-325-3p in bladder cancer cells.
Epithelial-mesenchymal transition (EMT) is a cellular program involved in embryogenesis, wound healing, and malignant progression. In cancers, EMT is frequently associated with tumor initiation, metastasis, and therapy resistance [27]. EMT is associated with inhibition of epithelial markers (E-cadherin) and tissue inhibitor of metalloproteinases-2 (TIMP-2), as well as the upregulation of mesenchymal markers (vimentin and N-cadherin) and mesenchymal marker of matrix metalloproteinase-9 (MMP-9) [28–31]. In the current study, expressions of E-cadherin, MMP-9, and TIMP-2 were detected to reflect the EMT state, and we found that MT3 promoted EMT of bladder cancer cells through reducing E-cadherin level and increasing TIMP-2 and MMP9 levels, thereby potentially increasing the migration and invasion ability of bladder cancer cells. Moreover, we found that miR-325-3p mimics conversely inhibited EMT in bladder cancer cells and compromised the effect of MT3 on EMT in bladder cancer cells. On one hand, we speculated that miR-325-3p regulated the EMT program through targeting MT3; on the other hand, the effects of miR-325-3p on cell invasion and migration were potentially attributed to the regulation of EMT by MT3. In addition, a study found that upregulation of MT3 is associated with poor prognosis in glioblastoma multiforme [32]. As EMT induction is significantly associated with high-grade and high-stage tumors [33], and high-grade tumors are more aggressive than low-grade ones, we speculated that miR-325-3p might also be correlated with bladder cancer grade, stage, and poor prognosis through MT3, but this needs further confirmation.
Conclusions
In conclusion, miR-325-3p expression is reduced in bladder cancer, and it regulates cell proliferation, migration, invasion, and EMT through targeting MT3. These findings provide new understanding of the roles of miR-325-3p and MT3 in bladder cancer and suggest a potential therapy.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Antoni S, Ferlay J. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71(1):96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Dobruch J, Daneshmand S, Fisch M, et al. Gender and bladder cancer: A collaborative review of etiology, biology, and outcomes. Eur Urol. 2016;69(2):300–10. doi: 10.1016/j.eururo.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–92. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 5.Roy S, Hooiveld Gj, Seehawer M, et al. microRNA 193a-5p regulates levels of nucleolar- and spindle-associated protein 1 to suppress hepatocarcinogenesis. Gastroenterology. 2018;155(6):1951–66.e26. doi: 10.1053/j.gastro.2018.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita K, Fujii T, Itami H, et al. NACC1, as a target of microRNA-331-3p regulates cell proliferation in urothelial carcinoma cells. Cancers (Basel) 2018;10(10) doi: 10.3390/cancers10100347. pii: E347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge Q, Lu M, Ju L, et al. miR-4324-RACGAP1-STAT3-ESR1 feedback loop inhibits proliferation and metastasis of bladder cancer. Int J Cancer. 2019;144(12):3043–55. doi: 10.1002/ijc.32036. [DOI] [PubMed] [Google Scholar]
- 8.Drayton Rm, Dudziec E, Peter S, et al. Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 2014;20(7):1990–2000. doi: 10.1158/1078-0432.CCR-13-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Zhuang J, Deng Y, et al. miR34a/GOLPH3 axis abrogates urothelial bladder cancer chemoresistance via reduced cancer stemness. Theranostics. 2017;7(19):4777–90. doi: 10.7150/thno.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Li Z, Soutto M, et al. Integrated analysis of mouse and human gastric neoplasms identifies conserved microRNA networks in gastric carcinogenesis. Gastroenterology. 2019;156(4):1127–39.e8. doi: 10.1053/j.gastro.2018.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Zhan Y, Chi J, et al. Using microRNAs as novel predictors of urologic cancer survival: An integrated analysis. EBioMedicine. 2018;34:94–107. doi: 10.1016/j.ebiom.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao S, Zhao T, Jin H. Expression of microRNA-325-3p and its potential functions by targeting HMGB1 in non-small cell lung cancer. Biomed Pharmacother. 2015;70:72–79. doi: 10.1016/j.biopha.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Han Y, Sun G, et al. MicroRNA-325-3p inhibits cell proliferation and induces apoptosis in hepatitis B virus-related hepatocellular carcinoma by down-regulation of aquaporin 5. Cell Mol Biol Lett. 2019;24:13. doi: 10.1186/s11658-019-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Lin T, Zhou S, Gao H, et al. MicroRNA-325 is a potential biomarker and tumor regulator in human bladder cancer. Technol Cancer Res Treat. 2018;17 doi: 10.1177/1533033818790536. 1533033818790536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Si M, Lang J. The roles of metallothioneins in carcinogenesis. J Hematol Oncol. 2018;11(1):107. doi: 10.1186/s13045-018-0645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomulkiewicz A, Jablonska K, Pula B, et al. Expression of metallothionein 3 in ductal breast cancer. Int J Oncol. 2016;49(6):2487–97. doi: 10.3892/ijo.2016.3759. [DOI] [PubMed] [Google Scholar]
- 17.Garrett Sh, Sens Ma, Shukla D, et al. Metallothionein isoform 3 expression in the human prostate and cancer-derived cell lines. Prostate. 1999;41(3):196–202. doi: 10.1002/(sici)1097-0045(19991101)41:3<196::aid-pros7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 18.Tsui KH, Hou CP, Chang KS, et al. Metallothionein 3 is a hypoxia-upregulated oncogene enhancing cell invasion and tumorigenesis in human bladder carcinoma cells. Int J Mol Sci. 2019;20(4) doi: 10.3390/ijms20040980. pii: E980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jafri MA, Al-Qahtani MH, Shay JW. Role of miRNAs in human cancer metastasis: Implications for therapeutic intervention. J Seminar Cancer Biol. 2017;44:117–31. doi: 10.1016/j.semcancer.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z, Li L, Du P, et al. Transcriptional downregulation of miR-4306 serves as a new therapeutic target for triple negative breast cancer. Theranostics. 2019;9(5):1401–16. doi: 10.7150/thno.30701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weidle Uh, Birzele F, Nopora A. MicroRNAs as potential targets for therapeutic intervention with metastasis of non-small cell lung cancer. J Cancer Genomics Proteomics. 2019;16(2):99–119. doi: 10.21873/cgp.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hydbring P, Wang Y, Fassl A, et al. Cell-cycle-targeting microRNAs as therapeutic tools against refractory cancers. Cancer Cell. 2017;31(4):576–90.e8. doi: 10.1016/j.ccell.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan P, Wu X, Liu X, et al. A causal relationship in spinal cord injury rat model between microglia activation and EGFR/MAPK detected by overexpression of microRNA-325-3p. J Mol Neurosci. 2019;68(2):181–90. doi: 10.1007/s12031-019-01297-w. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Sun B, Huang J, et al. Up-regulation of miR-325-3p suppresses pineal aralkylamine N-acetyltransferase (Aanat) after neonatal hypoxia-ischemia brain injury in rats. Brain Res. 2017;1668:28–35. doi: 10.1016/j.brainres.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Kmiecik Am, Pula B, Suchanski J, et al. Metallothionein-3 increases triple-negative breast cancer cell invasiveness via induction of metalloproteinase expression. PLoS One. 2015;10(5):e0124865. doi: 10.1371/journal.pone.0124865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao Yf, Xu Lx, Lu J, et al. Metallothionein III (MT3) is a putative tumor suppressor gene that is frequently inactivated in pediatric acute myeloid leukemia by promoter hypermethylation. J Transl Med. 2014;12:182. doi: 10.1186/1479-5876-12-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. J Trends Cell Biol. 2019;29(3):212–26. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 29.Reichl P, Dengler M, Van Zijl F, et al. Axl activates autocrine transforming growth factor-β signaling in hepatocellular carcinoma. Hepatology. 2015;61(3):930–41. doi: 10.1002/hep.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Y, Zhang Q, Zhao Q, et al. Tongxinluo attenuates myocardiac fibrosis after acute myocardial infarction in rats via inhibition of endothelial-to-mesenchymal transition. Biomed Res Int. 2019;2019 doi: 10.1155/2019/6595437. 6595437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ning X, Wang Y, Yan W, et al. Chitin synthesis inhibitors promote liver cancer cell metastasis via interfering with hypoxia-inducible factor 1α. Chemosphere. 2018;206:231–37. doi: 10.1016/j.chemosphere.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Mehrian-Shai R, Yalon M, Simon AJ, et al. High metallothionein predicts poor survival in glioblastoma multiforme. BMC Med Genomics. 2015;8:68. doi: 10.1186/s12920-015-0137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hensley PJ, Zetter D, Horbinski CM, et al. Association of epithelial-mesenchymal transition and nuclear cofilin with advanced urothelial cancer. J Human Pathol. 2016;57:68–77. doi: 10.1016/j.humpath.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]