Figure 1.
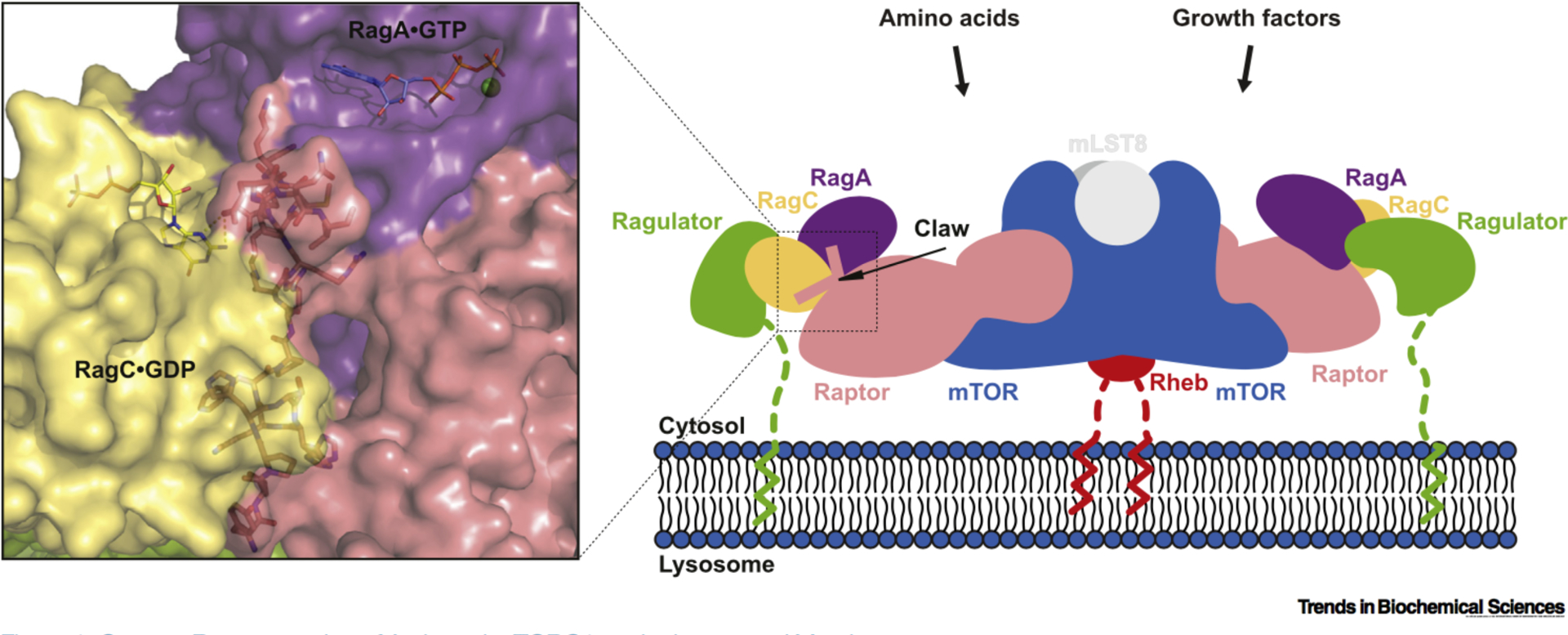
Cartoon Representation of Activated m TORC1 on the Lysosomal Membrane. mTOR kinase functions at the center of the cellular response to nutrient and growth factor availability, and controls metabolism, protein synthesis, and cell growth accordingly. Together with Raptor and mLST8, it forms the evolutionarily conserved signaling complex, mTORC1. Amino acids promote Rag GTPase–Ragulator-mediated translocation of mTORC1 to the lysosomal membrane via the myristoylated and palmitoylated 45 amino acid tail of Ragulator, enabling mTORC1 to be activated by growth factor-induced Rheb which is also localized to the membrane by a C-terminal famesyl group. The cryo-electron microscopy structure of the Raptor–Rag–Ragulator complex shows that Raptor selectively binds to the heterodimer of GTP-bound RagA and GOP-bound RagC via its own nucleotide detector, the ‘Raptor claw’, a triangular structure that threads between the GTPase domains of the Rag heterodimer (PDB 6U62). Abbreviations: mTORC1, mechanistic target of rapamycin complex 1.
