Abstract
Many airway diseases in children, notably bronchiolitis, cystic fibrosis (CF), non-CF bronchiectasis including primary ciliary dyskinesia, pneumonia, and severe asthma are associated with retention of airway secretions. Medications to improve secretions clearance, the mucoactive medications, are employed to treat these diseases with varying degrees of success. This manuscript reviews evidence for the use of these medications and future directions of study.
Abbreviations: AAP, American Academy of Pediatrics; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane ion regulator; ENaC, epithelial sodium channel; ERK, extracellular regulating kinase; HS, hyperosmolar saline; NAC, N-acetyl l-cysteine; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus; tPA, tissue plasminogen activator
Keywords: Sputum, Mucoactive medications, Mucolytics, Cystic fibrosis, Dornase, Airway diseases
Introduction: Definitions and taxonomy
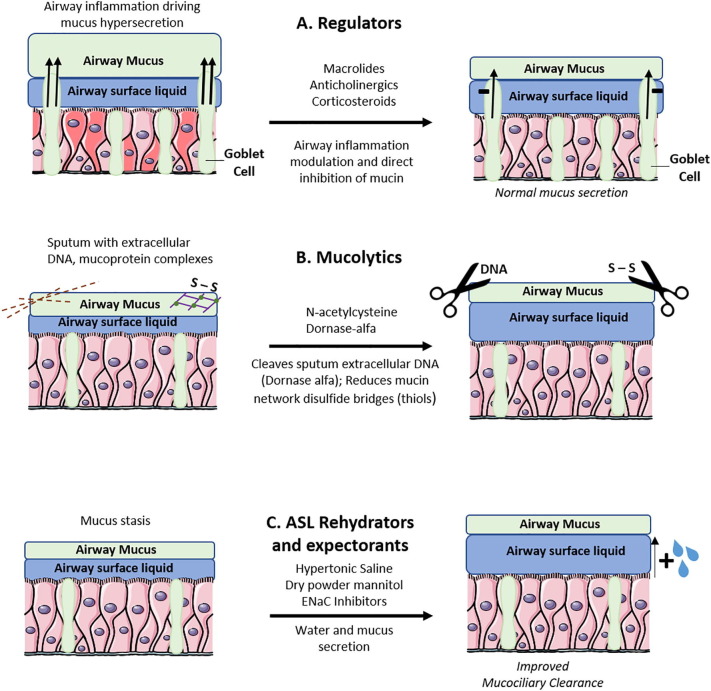
Normal mucus is the lining fluid that protects the airway by entrapping and clearing inhaled particulate matter, and prevents fluid loss from the airway surface. Normal mucus is comprised of approximately 95% water, with polymeric mucins particularly MUC5B secreted from submucosal glands and MUC5AC secreted from mucous or goblet cells, providing the gel structure [1], [2]. The mucous gel layer sits atop a periciliary layer of lower viscosity that contains attached mucins. The polymeric mucins form a gel through entanglement of long mucin chains. There is a relative paucity of cross-linking among discrete mucin polymers (Fig. 1 and Table 1 ).
Fig. 1.
(A) Regulators of goblet cell mucus secretion. Anticholinergics decrease mucus secretion by inhibition of neutrophil elastase driven mucin production (11). Anti-inflammatory agents such as corticosteroids and macrolides decrease mucus hypersecretion in airway inflammation. (B) Mucolytic agents break down mucus network structures to favorable biophysical properties for improved mucociliary clearance. Dornase-alfa hydrolyzes extracellular DNA in sputum and N-acetylcysteine reduces disulfide bonds in mucus networks. (C) Airway surface liquid (ASL) rehydrators reverse ASL height reduction to improve mucociliary clearance. These also increase mucus secretion as expectorators hypertonic saline and dry powder mannitol rehydrate ASL by hyperosmolar action. Epithelial sodium channel (ENaC) inhibitors prevent ASL depletion by hyperabsorption of sodium and water through ENaC.
Table 1.
Mucus therapies in cystic fibrosis lung disease management.
| Therapy | Mechanism | Form of Administration |
|---|---|---|
| Hypertonic Saline | Restoration of ASL Height | Aerosol mist |
| Mannitol | Restoration of ASL Height | Dry-power inhalation |
| ENaC Inhibitors | Restoration of ASL Height | N/A |
| Dornase alfa | DNA hydrolysis | Aerosol mist |
| N-acetylcysteine | Disulfide bond | Aerosol mist |
During infection and inflammation, airway secretions also contain inflammatory and shed epithelial cells, particulates, microorganisms, secreted peptides and products of inflammation. This complex mix is called phlegm when it is in the airway and sputum when expectorated [3]. Mucoactive drugs are meant to improve the clearance as well as decrease the production of phlegm. In some disorders, as discussed later, airways are obstructed by atypical secretions, such as lymphatic leakage as in plastic bronchitis. In some diseases, notably CF, reduction in the height of the periciliary fluid layer and increased adhesivity of the mucous gel can lead to poor mucus clearance causing accumulation within the airway that eventually can become infected phlegm.
Mucoactive medications are defined by their presumed mechanism of action [4]. The term mucolytic, a medication that breaks down polymer bonds within the secretions, has sometimes been incorrectly used interchangeably with the term mucoactive medication. Mucolytics can be classic mucolytics that break down mucins at the cross-linked disulfide bonds across adjacent cysteine residues. These classic mucolytics, of which N-acetyl L-cysteine (NAC) is the archetype, contain free sulfhydryl (thiol) groups that hydrolyze these disulfide bonds. Peptide mucolytics of which dornase alfa (Pulmozyme, Genentech, South San Francisco) is the archetype, depolymerize the secondary gel network comprised of polymeric DNA and filamentous (F-) actin. Because F-actin inhibits the effectiveness of dornase alfa, studies are underway evaluating the potential use of actin-protected dornase for more effective depolymerization of the DNA network (eg alidornase).
Expectorants are medications that increase the water content of secretions with the goal of improving clearance. One of the most widely studied of these is guaifenesin, but nearly all studies have shown guaifenesin and related compounds are no more effective than placebo [5]. Hyperosmolar medications such as hypertonic saline (HS) or dry powder mannitol are effective in increasing the hydration or fluid content of airway secretions and improving airway clearance in diseases such as CF and non-CF bronchiectasis [6], [7].
Mucokinetic medications are intended to improve the effectiveness of ciliary propulsion or cough in secretion clearance. Although beta-agonists such as salbutamol increase ciliary beat frequency, there is little evidence that they are effective mucokinetic drugs [8]. It could also be argued that by inducing effective coughing, hyperosmolar medications also have mucokinetic properties. Inhaled surfactant decreases the adhesivity of airway secretions, which potentially improves the effectiveness of ciliary and cough clearance [9].
Mucoregulatory medications decrease secretions by inhibiting mucus production or by decreasing inflammation. These medications include macrolide antibiotics that decrease mucin production by inhibiting the extracellular regulating kinase ERK1/2 [10], anticholinergic medications that may decrease mucus production by inhibiting neutrophil elastase driven mucin production [11], and corticosteroids which can decrease airway inflammation.
Mucospicic drugs (e.g. tetracycline) increase secretion viscosity. Secretions that are too thin might not be well cleared either by cough or by cilia, which is why mucus exists as a gel. Some patients, such as those with bronchorrhea, have extremely thin and liquid-like mucus. Mucospicic drugs may improve mucus clearance in these patients.
Viral bronchiolitis
Viral bronchiolitis is a clinical diagnosis in children characterized by obstructive dyspnea with increased respiratory effort, cough and - sometimes in young infants- apnea [12], [13]. It is most frequently caused by respiratory syncytial virus (RSV), but other respiratory viruses such as human metapneumovirus, bocavirus, human rhinovirus, para-influenza virus, coronavirus, enterovirus can be implicated as well [12], [13]. The pathobiology of bronchiolitis consists of edema of the small airways, increased local mucus production, and epithelial cell injury (necrosis and apoptosis) with ciliary dysfunction [14], [15]. The sloughing of dead airway cells together with an influx of leukocytes contributes to the thick mucus plugs that obstruct the smaller airways. Due to their small airways, especially young infants are prone to airway obstruction and at increased risk for hospitalization [16], [17], [18], [19]. Treatment for bronchiolitis is mainly supportive and as such there is need for an effective treatment targeting airway mucus obstruction.
The mucus plugs obstructing airways in viral bronchiolitis contain a large amount of polymerized, extracellular DNA [20]. As such, the use of the mucolytic dornase alfa, has been of interest [21], [22]. Two randomized, placebo-controlled studies of nebulized dornase alfa in hospitalized children with mild to moderate bronchiolitis and a Cochrane review did not show a decrease in length of hospital stay or respiratory effort [20], [23], [24]. However, one study did show an improvement in chest radiograph defined atelectasis compared to placebo in young children with severe bronchiolitis [20]. Although chest X-ray abnormalities as a single finding are clinically less relevant, this finding is consistent with case series of mechanically ventilated children that reported radiological and clinical improvement after the use of dornase alfa [25]. A more prominent role for mucus plugging in the severe cases of bronchiolitis is suggested. For example: large amounts of neutrophils have been shown to be present in the airway of children who were mechanically ventilated or died from severe bronchiolitis [26], [27], [28] and excessive formation of neutrophil extracellular traps may contribute to increased DNA content in mucus, further increasing its viscoelasticity [29], [30]. The current guideline from the American Academy of Pediatrics (AAP) on the treatment of bronchiolitis does not include a recommendation for using dornase alfa [31]. Future studies might focus on the use of dornase alfa in severe bronchiolitis and the possibilities for combined use with other aerosol agents to enhance small airway deposition.
The mucolytic NAC can be administered either by nebulization or orally, but has a low bioavailability as it undergoes first pass metabolism [32]. Although the use of NAC is carefully being explored for respiratory diseases in experimental settings, the clinical use of NAC for respiratory diseases is not recommended [33]. Despite one abstract reporting improvement of clinical severity scores when comparing nebulized NAC to salbutamol in children with viral bronchiolitis (full text article could not be retrieved), no studies have evaluated the role of NAC in viral bronchiolitis patients [34]. A possible beneficial effect for NAC can be hypothesized, as NAC functions as a free radical scavenger with anti-oxidative properties [35], [36]. Oxidative stress is implicated in the pathophysiology of bronchiolitis. Laboratory studies showed increased levels of intracellular H2O2 in pulmonary epithelial cells after RSV infection [37] and the addition of NAC to RSV infected airway cultures reduces MUC5AC expression in vitro [37].
Nebulized HS decreases mucus plugging through the osmotic hydration of mucus, improving mucociliary clearance [38] and stimulates effective cough through an irritant effect. The use of 3% HS has been extensively studied in viral bronchiolitis. An in 2017 updated Cochrane review on a total of 4195 children, showed that the use of nebulized hypertonic saline compared to normal (0.9%) saline resulted in a slightly shorter length of hospitalization, but also reported low quality evidence [39], [40]. Three randomized trials have been published since 2017. From them, only one study showed clinical improvement after three days of HS compared to one day of HS treatment but was underpowered [41], while two trials showed no beneficial effects for HS treatment compared to standard supportive care [42], [43]. In most studies, HS was administered in combination with bronchodilator agents because of the fear for HS induced bronchospasms. But a retrospective cohort study focusing on the use of 3% HS without bronchodilators reported only one bronchospasm among 377 included children [44]. In severe bronchiolitis, one retrospective study including 104 children admitted to pediatric intensive care unit (PICU) for mechanical ventilation reported both a decreased duration of respiratory support and length of PICU stay in favor of HS [45]. Again, this might implicate a more prominent role for mucus obstruction in the pathobiology of viral bronchiolitis in children necessitating PICU admission. The AAP guideline on the treatment of bronchiolitis takes a neutral position on this topic and does not give recommendations on the use of HS in hospitalized children [31].
Although children suffering from bronchiolitis may present with wheezing in the acute phase, the respiratory distress originates from to small airway inflammation with extensive mucus plugging and (in most cases) not from bronchospasms. Randomized controlled trials show no significant benefits from nebulized albuterol for bronchiolitis and a Cochrane review from 2014 reported no benefits in terms of oxygen saturation or clinical scores [46]. A single study evaluating the effects of epinephrine, levalbuterol, and racemic albuterol/salbutamol in mechanically ventilated children with bronchiolitis found a statistically significant, but clinically futile effect on airway peak pressures [47]. As such, the AAP advises against the use of bronchodilators for viral bronchiolitis [31] and a recent clinical study reporting no negatively affected clinical outcome after decreased use of albuterol affirms this recommendation [48].
Surfactant (mucokinetic) is essential for normal small airway function and prevention of alveolar collapse. In health, surfactant decreases the adhesive interaction between the cilia and mucus. This enables effective mucociliary clearance as it allows the cilia to beat without becoming entangled in the airway mucus [49]. A decreased amount of airway phosphatidylcholine and impaired surfactant function in children suffering from bronchiolitis is reported [50], [51]. This may be worse in severe cases of children who are in need of mechanical ventilation, as they suffer from more extensive airway inflammation [52]. A relationship between clinical recovery from bronchiolitis and improved surfactant activity is suggested [52]. To date, no clinical trials on the efficacy of exogenous surfactant administration in children hospitalized for mild to moderate bronchiolitis have been published. For children admitted to the PICU for bronchiolitis necessitating invasive ventilation, three randomized controlled trials have been published. A 2015 Cochrane review indicated favorable effect for duration of mechanical ventilation and PICU stay [52], [53], but only included a low number of subjects. The current AAP guideline does not include a recommendation regarding the use of nebulized surfactant for bronchiolitis [31].
Plastic bronchitis
Plastic bronchitis is a rare airway disorder defined by the presence of cohesive (rubbery) and branching casts that fill the airways [54]. This is different from mucus plugging where large, friable, non-branching phlegm contains mucin, inflammatory cells, and often bacteria. Although mucus plugs can be quite large, they do not have the extensive branching characteristics and rubbery texture of plastic bronchitis casts. They represent completely different etiologies. As one example, although extensive mucus plugging is common in patients with both CF and non-CF bronchiectasis, plastic bronchitis has never been reliably reported in patients with either of these disorders.
Plastic bronchitis is now identified as either being lymphatic or non-lymphatic in origin. The older classification systems that include inflammatory vs. non-inflammatory casts or Type 1 vs. Type 2 plastic bronchitis [55] were abandoned over 15 years ago when it was demonstrated that all forms of plastic bronchitis have some degree of inflammation. However, it is true that the non-lymphatic (or eosinophilic) plastic bronchitis has more inflammatory cells than classic or lymphatic plastic bronchitis [56].
Lymphatic plastic bronchitis appears to be the most common form of this disorder. It is most often reported in children and young adults with congenital heart disease, often after Fontan surgery and repair of single ventricle physiology. Plastic bronchitis is reported to occur in 3–4 % of these patients although the prevalence is probably under recognized [53]. As well described in another paper in this issue by Dr. Itkin and colleagues, there are characteristic abnormalities of the thoracic duct and lymphatic drainage that lead to plastic bronchitis when pulmonary vascular pressures are compromised.
Plastic bronchitis casts can be seen in some patients with sickle cell acute chest syndrome [57], primary lymphatic abnormalities, and following primary lymphatic malignancy. These lymphatic associated casts contain some cells, almost no fibrin, but extensive and congealed lymph. Because of this, classic mucolytics, peptide mucolytics, and fibrinolytic drugs are only partially effective in treating this disorder.
Eosinophilic plastic bronchitis produces branching casts that contain eosinophils and eosinophil degradation products including Charcot-Leyden crystals. Eosinophilic plastic bronchitis tends to occur in older children and young adults most of whom have a history of asthma and allergies [53]. These casts tend to occur in one branch of the airway and to reoccur only in that airway branch. Although this condition bears some resemblance to severe asthma where there is extensive mucus obstruction, even those patients have asthma tend to have relatively mild disease. In some respects, this disease is more like other eosinophilic diseases such as eosinophilic esophagitis.
It has been difficult to evaluate the effectiveness of mucoactive medications for the treatment of plastic bronchitis. There are relatively few subjects available for study in any one center leading to reports of individual case interventions. Also, the frequent confusion between plastic bronchitis and mucus plugging in those less familiar with these conditions makes it unclear which disorder has been treated.
Lymphatic plastic bronchitis does not respond to either classic or peptide mucolytics which should not be surprising as there appears to be little if any polymeric mucin or DNA in these casts. Tissue plasminogen activator (tPA) has been used acutely to degrade casts in patients with airway obstruction who are unable to tolerate bronchoscopy [58]. However, tPA should be considered a temporizing measure as it leads to extensive airway inflammation.
Tissue Factor activation has been associated with plastic bronchitis and so inhaled heparin has been used as chronic therapy to act both as an anti-inflammatory agent and to inhibit Tissue Factor. This therapy has met with variable success and appears to be most effective as a temporizing measure to prevent the formation of casts following bronchoscopic removal. There is no evidence for the effectiveness of inhaled hypertonic saline, NAC, steroids, salbutamol, or other medications for the treatment of lymphatic plastic bronchitis.
Eosinophilic or non-lymphatic plastic bronchitis often responds to corticosteroids administered in high dose or as pulse therapy. There is no proven effectiveness for the use of NAC, dornase alfa, hypertonic saline or other mucoactive medications for the treatment of non-lymphatic plastic bronchitis. Although it is possible that some of the newer biologics, particularly those that inhibit eosinophils, may be effective treatment of non-lymphatic plastic bronchitis, to our knowledge this has not been studied.
Cystic fibrosis and Non-Cystic fibrosis bronchiectasis
A major feature of cystic fibrosis lung disease is mucus accumulation resulting in airway obstruction. Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane ion regulator (CFTR) protein gene, resulting in decreased chloride and bicarbonate secretion at the apical cell membrane of airway epithelial cells. This decreased anion secretion, coupled with CFTR-mediated increased sodium absorption via the epithelial sodium channel (ENaC), results in higher partial osmotic pressure and compression of the airway surface liquid height [59]. This reduction of the airway surface liquid height results in reduced ciliary beat frequency and thus impaired mucociliary clearance, in turn resulting in mucus plug formation and small airway obstruction [59]. These changes in the airway mucus clearance, together with the primary CFTR defect, result in repeated cycles of infection and inflammation, bronchiectasis, and progressive loss of lung function [60]. Ameliorating these CFTR dysfunction related changes to airway mucus is thus an important therapeutic strategy in CF.
The first class of therapeutics addressing abnormal airway mucus in CF is those that directly hydrate the mucus. Depletion of the airway surface liquid occurs through decreased CFTR‐mediated fluid secretion with concomitant fluid absorption via the epithelial sodium channel (ENaC). Thus, one therapeutic strategy in CF lung disease is “rehydration” of the airway surface liquid by inhaling hyperosmolar agents. The premise of this class of therapies is that the airway surface liquid is pulled into the airway from the osmotic gradient generated [61]. The most widely used is aerosol hypertonic saline as a 7% sodium chloride solution [62]. Hypertonic saline, administered as an inhalation therapy twice daily, increases mucociliary clearance but not lung function in adults with CF [63] . In a double-blind, randomized control trial of inhaled hypertonic saline and isotonic saline in adults with CF, hypertonic saline failed to improve lung function but resulted in reduction of pulmonary exacerbations [64]. More recently, in the Saline Hypertonic in Preschoolers (SHIP) study, inhaled hypertonic saline was shown to improve the lung clearance Index, a marker of ventilation homogeneity, in children with CF aged 3–6 years [65]. Mannitol, which is a monosaccharide and is inhaled as a dry powder, has also been used in a similar fashion in CF and it improves lung function [66], [67] and surface properties of CF sputum [68]. In non-CF bronchiectasis, the role of inhaled hyperosmolar therapy is less clear. Hypertonic saline failed to improvements in exacerbation rate, lung function, and quality of life when compared to inhaled isotonic saline in a 12-month clinical trial [69]. Similarly, dry powder mannitol failed show improvement in function or respiratory symptom assessment over placebo [70] .
Another therapeutic strategy to rehydrate the airway surface liquid is targeting the epithelial sodium channel (ENaC). ENaC is an ion channel expressed on the apical surface of airway epithelial cells that conducts the resorption of sodium ions and water from the epithelial lumen into the epithelial cell [71]. In a CFTR deficient state there is evidence to suggest hyperabsorption of sodium and water via ENaC, which results in further depletion of the airway surface liquid [72]. There are ongoing efforts to develop ENaC inhibitors as potential therapeutics in CF to improve airway mucus and mucus clearance, though none are approved at the time of this review article.
CF airway mucus has abnormal viscoelastic properties [73] in part due to increased DNA and filamentous actin [74]. Another therapeutic strategy to improve airway mucus properties and enhance mucus clearance is to “thin” airway secretions with mucolytic and sputum-lytic agents. Dornase alfa has been widely adopted as an inhaled therapy in CF. Dornase alfa improves CF airway mucus properties by enzymatically degrading DNA, resulting in a decrease in mucus viscosity [21] and improvement in lung function [75]. While dornase alfa has been shown to be clinically beneficial in CF lung disease, it has been shown to be harmful in non-CF bronchiectasis, with increased risk of exacerbations and decrease in lung function when compared to placebo [76]. Another proposed mucolytic therapy in CF is N-acetylcysteine, which disrupts the mucus architecture through hydrolysis of the disulfide bonds tethering mucus strands. However, there are no studies to date to show clinical benefit in CF lung disease [77].
Asthma
Asthma is a common, chronic inflammatory disorder of the airways that is primarily associated with bronchial hyperresponsiveness and intermittent airflow obstruction. Mucus hypersecretion and airway obstruction by mucus plugs often contribute to disease symptoms, in particular during exacerbations and especially with severe, life-threatening or fatal asthma attacks [78], [79], [80]. Current, well-established, asthma medications, including both controller and reliever therapies such as 2-agonists [81], [82], [83], anticholinergics [32], [84], [85], [86], and corticosteroids [87] have putative mucoregulatory activities. However, in the clinical evaluation of these agents, any direct mucoregulatory effects (e.g. reduction of mucus secretion or occurrence of mucus plugs) will be difficult to distinguish from their other more primary bronchodilator and/or immunomodulatory functions. On the other hand, macrolides, have been shown to decrease mucus secretion both in vitro and in vivo [88], and may have a role as an anti-inflammatory to treat asthma, but their clinical benefit in both children and adults with chronic asthma has not been established [89].
Mucolytic agents, including dornase alfa and NAC, are not used in the management of asthma. There is no evidence of a clinical benefit of dornase alfa in children [90] or adults [91] with moderate to severe asthma. Nevertheless, some clinicians appear to use dornase alfa in mechanically ventilated children,[92] fueled by case reports showing improvement agent [93], [94]. The administration of NAC in asthma has met with very limited success, as more recently assessed in a systematic review and meta-analysis.[95] Although expectorants, such as hypertonic saline, which is frequently used for sputum induction during bronchial challenge, have been shown to improve mucociliary and cough clearance in asthmatics in small studies, [96], [97] these agents are currently not recommended in asthma management and indeed are known to induce mucus secretion and bronchospasm.
Although these findings reduce the likelihood that mucoactive agents in current use will play a role in the pharmacological approach to (pediatric) asthma, increasing insight into asthma endotypes (e.g. allergic, non-allergic, neutrophilic, cough-dominant etc), in which different immune cells (e.g. eosinophils vs neutrophils) function as the primary drivers of asthma pathophysiology, may establish the relative importance of mucus in defining clinical signs of illness, particularly in T17 dominant (non-eosinophilic) asthma. As such, the concept of secretory hyperresponsiveness, which has strong links to IL-13 and neutrophil elastase mediated responses, [11], [98], [99] may serve as an anchor point for the use of mucoactive agents in asthma.
Pediatric critical care: Mechanically ventilated children
Children with acute respiratory failure undergoing invasive mechanical ventilation in the pediatric intensive care unit (PICU) comprise a patient group highly vulnerable for airway obstruction by phlegm. Airway obstruction in these children increases the risk of atelectasis, ventilator-associated pneumonia and need for high peak airway pressures. At the same time, an indwelling endotracheal tube provides an opportunity for new local drug delivery for mucoactive agents [100], [101].
Disappointingly, few randomized controlled studies have so far studied the effectiveness of mucoactive agents during invasive mechanical ventilation in an either specific or general PICU population. McKinley et al. have investigated the effect of no saline versus 0.225% or 0.9% saline installation before routine endotracheal suction to remove phlegm in 427 children receiving ventilator support [102]. However, in this non-blinded trial, no statistically significant differences in duration of mechanical ventilation, oxygen therapy or PICU stay between the groups were found. Similarly, Shein et al. have focused on expectorants by studying routine nebulization with hypertonic (3%) saline versus normal (0.9%) saline in a pilot study [103]. With a very small sample size (n = 18), they found no differences between the groups for a number of outcomes, including duration of mechanical ventilation or chest X-ray atelectasis score, as well as respiratory mechanics parameters before and after intervention. Finally, Riethmueller and co-workers studied the mucolytic agent aldornasfa versus normal saline in 88 mechanically ventilated children with an age of 0–2 years after cardiac surgery [104]. Nebulization with dornase alfa resulted in a statistically significant reduction in duration of ventilator support by approximately one day, as well as a trend (p = 0.051) towards improved chest X-ray atelectasis scores. However, the primary endpoint of this study, re-intubation incidence, was not significantly different between the groups.
These results do not make any recommendations on routine mucoactive medication strategies for PICU patients possible. They merely reveal the need for further randomized trials in this field. With this, we need to realize that subgroups of PICU patients either with or without active respiratory inflammation and infection may react differently to expectorants versus pure mucolytics. As such, better insight into the dynamics of mucus and phlegm viscoelastic properties and contents (e.g. extracellular DNA, proteins) during the course of mechanical ventilation for pediatric critical illnesses is highly relevant.
Conclusion & future research directions
A characteristic of many airway diseases is secretion retention. Although various mucoactive medications, with diverse mechanisms of action, have been studied as potential therapies, there are few well controlled clinical studies supporting their use; especially for older medications. There has been a renewed interest in testing existing drugs and developing new medications targeting specific abnormalities in mucus clearance. This is driven, in large part, by a better understanding of the importance of secretion clearance and the mechanism(s) of action of mucoactive medications as well as a better understanding of how mucus secretion and clearance changes with disease type and disease activity.
Educational aims
The reader will be able to:
-
•
Understand the classification of mucoactive medications based upon their proposed mechanism of action
-
•
Identify medications that have been approved for secretion clearance therapy and their specific indications
-
•
Appreciate how to evaluate the effectiveness of mucoactive medications for different airway diseases
-
•
Recognize that medications that are effective for one disease may not be effective or may even be harmful for treating other diseases.
Author contributions
Linssen, RSN: contributed to the writing of the manuscript.
Ma, J: contributed to the writing of the manuscript.
Bem, RA: contributed to the writing of the manuscript.
Rubin, BK: contributed to the design of the study, contributed to the writing of the manuscript.
Acknowledgments
Acknowledgements
None.
Funding
None.
Declarations of interest
None.
References
- 1.Voynow J.A., Rubin B.K. Mucins, mucus, and sputum. Chest. 2009;135(2):505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 2.Ma J., Rubin B.K., Voynow J.A. Mucins, mucus, and goblet cells. Chest. 2018;154(1):169–176. doi: 10.1016/j.chest.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Rubin B.K. Mucus, phlegm, and sputum in cystic fibrosis. Respir Care. 2009;54(6):726–732. doi: 10.4187/002013209790983269. discussion 32. [DOI] [PubMed] [Google Scholar]
- 4.Bk R. Marcel Dekker, Inc; New York: 2004. Taxonomy of mucoactive medications. [Google Scholar]
- 5.Hoffer-Schaefer A., Rozycki H.J., Yopp M.A., Rubin B.K. Guaifenesin has no effect on sputum volume or sputum properties in adolescents and adults with acute respiratory tract infections. Respir Care. 2014;59(5):631–636. doi: 10.4187/respcare.02640. [DOI] [PubMed] [Google Scholar]
- 6.Nevitt S.J., Thornton J., Murray C.S., Dwyer T. Inhaled mannitol for cystic fibrosis. Cochrane Database Syst Rev. 2020;5(5)::Cd008649. doi: 10.1002/14651858.CD008649.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wark P., McDonald V.M. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev. 2018;9(9):Cd001506. doi: 10.1002/14651858.CD001506. [DOI] [PubMed] [Google Scholar]
- 8.Isawa T., Teshima T., Hirano T., Ebina A., Anazawa Y., Konno K. Effect of bronchodilation on the deposition and clearance of radioaerosol in bronchial asthma in remission. J Nuclear Med. 1987;28(12):1901–1906. [PubMed] [Google Scholar]
- 9.Anzueto A., Jubran A., Ohar J.A., Piquette C.A., Rennard S.I., Colice G. Effects of aerosolized surfactant in patients with stable chronic bronchitis: a prospective randomized controlled trial. JAMA. 1997;278(17):1426–1431. [PubMed] [Google Scholar]
- 10.Shinkai M., Foster G.H., Rubin B.K. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L75–L85. doi: 10.1152/ajplung.00093.2005. [DOI] [PubMed] [Google Scholar]
- 11.Komiya K., Kawano S., Suzaki I., Akaba T., Kadota J.I., Rubin B.K. Tiotropium inhibits mucin production stimulated by neutrophil elastase but not by IL-13. Pulm Pharmacol Ther. 2018;48:161–167. doi: 10.1016/j.pupt.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Meissner H.C. Viral bronchiolitis in children. N Engl J Med. 2016;374(1):62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 13.Florin T.A., Plint A.C., Zorc J.J. Viral bronchiolitis. The Lancet. 2017;389(10065):211–224. doi: 10.1016/S0140-6736(16)30951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickles R.J., DeVincenzo J.P. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J Pathol. 2015;235(2):266–276. doi: 10.1002/path.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bem R.A., Domachowske J.B., Rosenberg H.F. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol. 2011;301(2):L148–L156. doi: 10.1152/ajplung.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terry P.B., Traystman R.J. The clinical significance of collateral ventilation. Ann Am Thorac Soc. 2016;13(12):2251–2257. doi: 10.1513/AnnalsATS.201606-448FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall C.B., Weinberg G.A., Blumkin A.K., Edwards K.M., Staat M.A., Schultz A.F. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132(2):e341–e348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 18.Hall C.B., Weinberg G.A., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M.A. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujiogi M, Goto T, Yasunaga H, Fujishiro J, Mansbach JM, Camargo CA, Jr., et al. Trends in Bronchiolitis Hospitalizations in the United States: 2000-2016. Pediatrics. 2019;144(6). [DOI] [PMC free article] [PubMed]
- 20.Nasr S.Z., Strouse P.J., Soskolne E., Maxvold N.J., Garver K.A., Rubin B.K. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest. 2001;120(1):203–208. doi: 10.1378/chest.120.1.203. [DOI] [PubMed] [Google Scholar]
- 21.Shak S., Capon D.J., Hellmiss R., Marsters S.A., Baker C.L. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. PNAS. 1990;87(23):9188–9192. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs H.J., Borowitz D.S., Christiansen D.H., Morris E.M., Nash M.L., Ramsey B.W. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 23.Boogaard R., Hulsmann A.R., van Veen L., Vaessen-Verberne A., Yap Y.N., Sprij A.J. Recombinant human deoxyribonuclease in infants with respiratory syncytial virus bronchiolitis. Chest. 2007;131(3):788–795. doi: 10.1378/chest.06-2282. [DOI] [PubMed] [Google Scholar]
- 24.Enriquez A., Chu I.W., Mellis C., Lin W.Y. Nebulised deoxyribonuclease for viral bronchiolitis in children younger than 24 months. Cochrane Database Syst Rev. 2012;11:CD008395. doi: 10.1002/14651858.CD008395.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merkus P.J., de Hoog M., van Gent R., de Jongste J.C. DNase treatment for atelectasis in infants with severe respiratory syncytial virus bronchiolitis. Eur Respir J. 2001;18(4):734–737. [PubMed] [Google Scholar]
- 26.Mod Pathol. 2007;20(1):108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 27.Geerdink R.J., Hennus M.P., Westerlaken G.H.A., Abrahams A.C., Albers K.I., Walk J. LAIR-1 limits neutrophil extracellular trap formation in viral bronchiolitis. J Allergy Clin Immunol. 2018;141(2):811–814. doi: 10.1016/j.jaci.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 28.Cortjens B, Ingelse SA, Calis JC, Vlaar AP, Koenderman L, Bem RA, et al. Neutrophil subset responses in infants with severe viral respiratory infection. (1521-7035 (Electronic)). [DOI] [PubMed]
- 29.Cortjens B, de Boer OJ, de Jong R, Antonis AF, Sabogal Pineros YS, Lutter R, et al. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. (1096-9896 (Electronic)). [DOI] [PubMed]
- 30.Cortjens B, de Jong R, Bonsing JG, van Woensel JBM, Antonis AFG, Bem RA. Local dornase alfa treatment reduces NETs-induced airway obstruction during severe RSV infection. (1468-3296 (Electronic)). [DOI] [PubMed]
- 31.Ralston S.L., Lieberthal A.S., Meissner H.C., Alverson B.K., Baley J.E., Gadomski A.M. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 32.Balsamo R., Lanata L., Egan C.G. Mucoactive drugs. Eur Respir Rev. 2010;19(116):127–133. doi: 10.1183/09059180.00003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee S, McCormack S. Acetylcysteine for Patients Requiring Mucous Secretion Clearance: A Review of Clinical Effectiveness and Safety. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health Copyright (c) 2019 Canadian Agency for Drugs and Technologies in Health.; 2019. [PubMed]
- 34.Naz F., Raza A.B., Ijaz I., Kazi M.Y. Effectiveness of nebulized N-acetylcysteine solution in children with acute bronchiolitis. J College Phys Surg-Pakistan: JCPSP. 2014;24(6):408–411. [PubMed] [Google Scholar]
- 35.Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 2018;52(7):751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 36.Rubin B.K. Aerosol medications for treatment of mucus clearance disorders. Respir Care. 2015;60(6) doi: 10.4187/respcare.04087. [DOI] [PubMed] [Google Scholar]
- 37.Mata M., Morcillo E., Gimeno C., Cortijo J. N-acetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV) Biochem Pharmacol. 2011;82(5):548–555. doi: 10.1016/j.bcp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Mandelberg A., Amirav I. Hypertonic saline or high volume normal saline for viral bronchiolitis: mechanisms and rationale. Pediatr Pulmonol. 2010;45(1):36–40. doi: 10.1002/ppul.21185. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L., Mendoza-Sassi R.A., Wainwright C., Klassen T.P. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2017;12:CD006458. doi: 10.1002/14651858.CD006458.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison W., Angoulvant F., House S., Gajdos V., Ralston S.L. Hypertonic saline in bronchiolitis and type I Error: A trial sequential analysis. Pediatrics. 2018;142(3) doi: 10.1542/peds.2018-1144. [DOI] [PubMed] [Google Scholar]
- 41.Beal G., Barbier C., Thoret S., Rubio A., Bonnet M., Mazet R. Nebulized hypertonic saline 3% for 1 versus 3 days in hospitalized bronchiolitis: a blinded non-inferiority randomized controlled trial. BMC Pediatr. 2019;19(1):417. doi: 10.1186/s12887-019-1804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morikawa Y., Miura M., Furuhata M.Y., Morino S., Omori T., Otsuka M. Nebulized hypertonic saline in infants hospitalized with moderately severe bronchiolitis due to RSV infection: A multicenter randomized controlled trial. Pediatr Pulmonol. 2018;53(3):358–365. doi: 10.1002/ppul.23945. [DOI] [PubMed] [Google Scholar]
- 43.Jaquet-Pilloud R., Verga M.E., Russo M., Gehri M., Pauchard J.Y. Nebulised hypertonic saline in moderate-to-severe bronchiolitis: a randomised clinical trial. Arch Dis Child. 2020;105(3):236–240. doi: 10.1136/archdischild-2019-317160. [DOI] [PubMed] [Google Scholar]
- 44.Bhilare N.V., Dhaneshwar S.S., Sinha A.J., Kandhare A.D., Bodhankar S.L. Novel thioester prodrug of N-acetylcysteine for odor masking and bioavailability enhancement. Curr Drug Deliv. 2016;13(4):611–620. doi: 10.2174/1567201812666150904144607. [DOI] [PubMed] [Google Scholar]
- 45.Stobbelaar K., Kool M., de Kruijf D., Van Hoorenbeeck K., Jorens P., De Dooy J. Nebulised hypertonic saline in children with bronchiolitis admitted to the paediatric intensive care unit: A retrospective study. J Paediatr Child Health. 2019;55(9):1125–1132. doi: 10.1111/jpc.14371. [DOI] [PubMed] [Google Scholar]
- 46.Gadomski A.M., Scribani M.B. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2014;6:CD001266. doi: 10.1002/14651858.CD001266.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levin D.L., Garg A., Hall L.J., Slogic S., Jarvis J.D., Leiter J.C. A prospective randomized controlled blinded study of three bronchodilators in infants with respiratory syncytial virus bronchiolitis on mechanical ventilation. Pediatric Critic Care Med. 2008;9(6):598–604. doi: 10.1097/PCC.0b013e31818c82b4. [DOI] [PubMed] [Google Scholar]
- 48.Dunn M., Muthu N., Burlingame C.C., Gahman A.M., McCloskey M., Tyler L.M. Reducing albuterol use in children with bronchiolitis. Pediatrics. 2020;145(1) doi: 10.1542/peds.2019-0306. [DOI] [PubMed] [Google Scholar]
- 49.Girod S., Galabert C., Lecuire A., Zahm J.M., Puchelle E. Phospholipid composition and surface-active properties of tracheobronchial secretions from patients with cystic fibrosis and chronic obstructive pulmonary diseases. Pediatr Pulmonol. 1992;13(1):22–27. doi: 10.1002/ppul.1950130107. [DOI] [PubMed] [Google Scholar]
- 50.Skelton R., Darowski P.H.M., Chetcuti P.A., Morgan L.W., Harwood J.L. Abnormal surfactant composition and activity in severe bronchiolitis. Acta Paediatr. 1999;88:942–946. doi: 10.1080/08035259950168414. [DOI] [PubMed] [Google Scholar]
- 51.LeVine A.M., Lotze A., Stanley S., Stroud C., O'Donnell R., Whitsett J. Surfactant content in children with inflammatory lung disease. Crit Care Med. 1996;24(6):1062–1067. doi: 10.1097/00003246-199606000-00029. [DOI] [PubMed] [Google Scholar]
- 52.Jat K.R., Chawla D. Surfactant therapy for bronchiolitis in critically ill infants. Cochrane Database Syst Rev. 2015;8:CD009194. doi: 10.1002/14651858.CD009194.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davison C., Ventre K.M., Luchetti M., Randolph A.G. Efficacy of interventions for bronchiolitis in critically ill infants: a systematic review and meta-analysis. Pediatric Critical Care Med. 2004;5(5):482–489. doi: 10.1097/01.pcc.0000128891.54799.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubin BK IM. Plastic Bronchitis. 2nd ed., 2019.
- 55.Seear M., Hui H., Magee F., Bohn D., Cutz E. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med. 1997;155(1):364–370. doi: 10.1164/ajrccm.155.1.9001337. [DOI] [PubMed] [Google Scholar]
- 56.Madsen P., Shah S.A., Rubin B.K. Plastic bronchitis: new insights and a classification scheme. Paediatr Respir Rev. 2005;6(4):292–300. doi: 10.1016/j.prrv.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Moser C., Nussbaum E., Cooper D.M. Plastic bronchitis and the role of bronchoscopy in the acute chest syndrome of sickle cell disease. Chest. 2001;120(2):608–613. doi: 10.1378/chest.120.2.608. [DOI] [PubMed] [Google Scholar]
- 58.Gibb E., Blount R., Lewis N., Nielson D., Church G., Jones K. Management of plastic bronchitis with topical tissue-type plasminogen activator. Pediatrics. 2012;130(2):e446–e450. doi: 10.1542/peds.2011-2883. [DOI] [PubMed] [Google Scholar]
- 59.Boucher R.C. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58(1):157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 60.Fahy J.V., Dickey B.F. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mall M.A. Unplugging mucus in cystic fibrosis and chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(Suppl. 2):S177–S185. doi: 10.1513/AnnalsATS.201509-641KV. [DOI] [PubMed] [Google Scholar]
- 62.Robinson M., Regnis J.A., Bailey D.L., King M., Bautovich G.J., Bye P.T. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. Am J Respir Crit Care Med. 1996;153(5):1503–1509. doi: 10.1164/ajrccm.153.5.8630593. [DOI] [PubMed] [Google Scholar]
- 63.Donaldson S.H., Bennett W.D., Zeman K.L., Knowles M.R., Tarran R., Boucher R.C. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354(3):241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 64.Elkins M.R., Robinson M., Rose B.R., Harbour C., Moriarty C.P., Marks G.B. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 65.Ratjen F., Davis S.D., Stanojevic S., Kronmal R.A., Hinckley Stukovsky K.D., Jorgensen N. Inhaled hypertonic saline in preschool children with cystic fibrosis (SHIP): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2019;7(9):802–809. doi: 10.1016/S2213-2600(19)30187-0. [DOI] [PubMed] [Google Scholar]
- 66.Jaques A., Daviskas E., Turton J.A., McKay K., Cooper P., Stirling R.G. Inhaled mannitol improves lung function in cystic fibrosis. Chest. 2008;133(6):1388–1396. doi: 10.1378/chest.07-2294. [DOI] [PubMed] [Google Scholar]
- 67.Aitken M.L., Bellon G., De Boeck K., Flume P.A., Fox H.G., Geller D.E. Long-term inhaled dry powder mannitol in cystic fibrosis: an international randomized study. Am J Respir Crit Care Med. 2012;185(6):645–652. doi: 10.1164/rccm.201109-1666OC. [DOI] [PubMed] [Google Scholar]
- 68.Daviskas E., Anderson S.D., Jaques A., Charlton B. Inhaled mannitol improves the hydration and surface properties of sputum in patients with cystic fibrosis. Chest. 2010;137(4):861–868. doi: 10.1378/chest.09-2017. [DOI] [PubMed] [Google Scholar]
- 69.Nicolson C.H., Stirling R.G., Borg B.M., Button B.M., Wilson J.W., Holland A.E. The long term effect of inhaled hypertonic saline 6% in non-cystic fibrosis bronchiectasis. Respir Med. 2012;106(5):661–667. doi: 10.1016/j.rmed.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 70.Bilton D., Daviskas E., Anderson S.D., Kolbe J., King G., Stirling R.G. Phase 3 randomized study of the efficacy and safety of inhaled dry powder mannitol for the symptomatic treatment of non-cystic fibrosis bronchiectasis. Chest. 2013;144(1):215–225. doi: 10.1378/chest.12-1763. [DOI] [PubMed] [Google Scholar]
- 71.Hanukoglu I., Hanukoglu A. Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene. 2016;579(2):95–132. doi: 10.1016/j.gene.2015.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Althaus M. ENaC inhibitors and airway re-hydration in cystic fibrosis: state of the art. Current Mol Pharmacol. 2013;6(1):3–12. doi: 10.2174/18744672112059990025. [DOI] [PubMed] [Google Scholar]
- 73.Ma J.T., Tang C., Kang L., Voynow J.A., Rubin B.K. Cystic fibrosis sputum rheology correlates with both acute and longitudinal changes in lung function. Chest. 2018;154(2):370–377. doi: 10.1016/j.chest.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 74.Henke M.O., Ratjen F. Mucolytics in cystic fibrosis. Paediatr Respir Rev. 2007;8(1):24–29. doi: 10.1016/j.prrv.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 75.Aitken M.L., Burke W., McDonald G., Shak S., Montgomery A.B., Smith A. Recombinant human DNase inhalation in normal subjects and patients with cystic fibrosis. A phase 1 study. JAMA. 1992;267(14):1947–1951. [PubMed] [Google Scholar]
- 76.O'Donnell A.E., Barker A.F., Ilowite J.S., Fick R.B. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest. 1998;113(5):1329–1334. doi: 10.1378/chest.113.5.1329. [DOI] [PubMed] [Google Scholar]
- 77.YCM Duijvestijn PLP Brand Systematic review of N-acetylcysteine in cystic fibrosis 88 1 1999 38 41 10.1111/apa.1999.88.issue-1 10.1111/j.1651-2227.1999.tb01265.x http://doi.wiley.com/10.1111/apa.1999.88.issue-1 http://doi.wiley.com/10.1111/j.1651-2227.1999.tb01265.x. [DOI] [PubMed]
- 78.Rubin B.K., Tomkiewicz R., Fahy J.V., Green F.H. Histopathology of fatal asthma: drowning in mucus. Pediatr Pulmonol. 2001;(Suppl 23):88–89. doi: 10.1002/ppul.1950230851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rogers D.F. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004;4(3):241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 80.Kuyper L.M., Pare P.D., Hogg J.C., Lambert R.K., Ionescu D., Woods R. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115(1):6–11. doi: 10.1016/s0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 81.Delavoie F., Molinari M., Milliot M., Zahm J.M., Coraux C., Michel J. Salmeterol restores secretory functions in cystic fibrosis airway submucosal gland serous cells. Am J Respir Cell Mol Biol. 2009;40(4):388–397. doi: 10.1165/rcmb.2008-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson M., Rennard S. Alternative mechanisms for long-acting beta(2)-adrenergic agonists in COPD. Chest. 2001;120(1):258–270. doi: 10.1378/chest.120.1.258. [DOI] [PubMed] [Google Scholar]
- 83.Lazarus S.C., Basbaum C.B., Gold W.M. Localization of cAMP in dog and cat trachea: effects of beta-adrenergic agonists. Am J Physiol. 1984;247(5 Pt 1):C327–C334. doi: 10.1152/ajpcell.1984.247.5.C327. [DOI] [PubMed] [Google Scholar]
- 84.Arai N., Kondo M., Izumo T., Tamaoki J., Nagai A. Inhibition of neutrophil elastase-induced goblet cell metaplasia by tiotropium in mice. Eur Respir J. 2010;35(5):1164–1171. doi: 10.1183/09031936.00040709. [DOI] [PubMed] [Google Scholar]
- 85.Meltzer E.O. Intranasal anticholinergic therapy of rhinorrhea. J Allergy Clin Immunol. 1992;90(6 Pt 2):1055–1064. doi: 10.1016/0091-6749(92)90123-j. [DOI] [PubMed] [Google Scholar]
- 86.Yuta A., Baraniuk J.N. Therapeutic approaches to mucus hypersecretion. Curr Allergy Asthma Rep. 2005;5(3):243–251. doi: 10.1007/s11882-005-0044-6. [DOI] [PubMed] [Google Scholar]
- 87.Agnew J.E., Bateman J.R., Pavia D., Clarke S.W. Peripheral airways mucus clearance in stable asthma is improved by oral corticosteroid therapy. Bull Eur Physiopathol Respirat. 1984;20(3):295–301. [PubMed] [Google Scholar]
- 88.Kanoh S., Rubin B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23(3):590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kew K.M., Undela K., Kotortsi I., Ferrara G. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015;9:CD002997. doi: 10.1002/14651858.CD002997.pub4. [DOI] [PubMed] [Google Scholar]
- 90.Boogaard R., Smit F., Schornagel R., Vaessen-Verberne A.A., Kouwenberg J.M., Hekkelaan M. Recombinant human deoxyribonuclease for the treatment of acute asthma in children. Thorax. 2008;63(2):141–146. doi: 10.1136/thx.2007.081703. [DOI] [PubMed] [Google Scholar]
- 91.Silverman R.A., Foley F., Dalipi R., Kline M., Lesser M. The use of rhDNAse in severely ill, non-intubated adult asthmatics refractory to bronchodilators: a pilot study. Respir Med. 2012;106(8):1096–1102. doi: 10.1016/j.rmed.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Snoek A.P., Brierley J. Mucolytics for intubated asthmatic children: a national survey of United kingdom paediatric intensive care consultants. Crit Care Res Pract. 2015;2015 doi: 10.1155/2015/396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel A., Harrison E., Durward A., Murdoch I.A. Intratracheal recombinant human deoxyribonuclease in acute life-threatening asthma refractory to conventional treatment. Br J Anaesth. 2000;84(4):505–507. doi: 10.1093/oxfordjournals.bja.a013479. [DOI] [PubMed] [Google Scholar]
- 94.Durward A., Forte V., Shemie S.D. Resolution of mucus plugging and atelectasis after intratracheal rhDNase therapy in a mechanically ventilated child with refractory status asthmaticus. Crit Care Med. 2000;28(2):560–562. doi: 10.1097/00003246-200002000-00045. [DOI] [PubMed] [Google Scholar]
- 95.Tarrant B.J., Le Maitre C., Romero L., Steward R., Button B.M., Thompson B.R. Mucoactive agents for chronic, non-cystic fibrosis lung disease: A systematic review and meta-analysis. Respirology. 2017;22(6):1084–1092. doi: 10.1111/resp.13047. [DOI] [PubMed] [Google Scholar]
- 96.Daviskas E., Anderson S.D., Eberl S., Young I.H. Beneficial effect of inhaled mannitol and cough in asthmatics with mucociliary dysfunction. Respir Med. 2010;104(11):1645–1653. doi: 10.1016/j.rmed.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 97.Daviskas E., Anderson S.D., Gonda I., Eberl S., Meikle S., Seale J.P. Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects. Eur Respir J. 1996;9(4):725–732. doi: 10.1183/09031936.96.09040725. [DOI] [PubMed] [Google Scholar]
- 98.Kanoh S., Tanabe T., Rubin B.K. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Experiment Allergy. 2011;41(12):1747–1756. doi: 10.1111/j.1365-2222.2011.03852.x. [DOI] [PubMed] [Google Scholar]
- 99.Rubin B.K., Priftis K.N., Schmidt H.J., Henke M.O. Secretory hyperresponsiveness and pulmonary mucus hypersecretion. Chest. 2014;146(2):496–507. doi: 10.1378/chest.13-2609. [DOI] [PubMed] [Google Scholar]
- 100.Krause M.F., von Bismarck P., Oppermann H.C., Ankermann T. Bronchoscopic surfactant administration in pediatric patients with persistent lobar atelectasis. Respiration. 2008;75(1):100–104. doi: 10.1159/000088713. [DOI] [PubMed] [Google Scholar]
- 101.Hendriks T., de Hoog M., Lequin M.H., Devos A.S., Merkus P.J. DNase and atelectasis in non-cystic fibrosis pediatric patients. Critical Care (London, England) 2005;9(4):R351–R356. doi: 10.1186/cc3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McKinley D.F., Kinney S.B., Copnell B., Shann F. Long-term effects of saline instilled during endotracheal suction in pediatric intensive care: a randomized trial. Am J Crit Care. 2018;27(6):486–494. doi: 10.4037/ajcc2018615. [DOI] [PubMed] [Google Scholar]
- 103.Shein S.L., Gallagher J.T., Deakins K.M., Weinert D.M. Prophylactic use of nebulized hypertonic saline in mechanically ventilated children: a randomized blinded pilot study. Respir Care. 2016;61(5):586–592. doi: 10.4187/respcare.04418. [DOI] [PubMed] [Google Scholar]
- 104.Riethmueller J., Borth-Bruhns T., Kumpf M., Vonthein R., Wiskirchen J., Stern M. Recombinant human deoxyribonuclease shortens ventilation time in young, mechanically ventilated children. Pediatr Pulmonol. 2006;41(1):61–66. doi: 10.1002/ppul.20298. [DOI] [PubMed] [Google Scholar]