Abstract
Regenerative medicine represents a major challenge for the scientific community. The choice of the biological sources used, such as stem cells and grafts, is crucial. Stem cell therapy is mainly related to the use of mesenchymal stem cells; however, clinical trials are still needed to investigate their safety. The micrografting technique was conceived by Cicero Parker Meek in 1958. It is based on the principle that by increasing the superficial area of skin grafts and reducing the size of its particles, it is possible to cover an area larger than the original donor site. Stem cells are pluripotent cells that have the capacity to differentiate into all cell types and are self-renewing, whereas micrografts derive from a small fragment of an autologous tissue and exhibit limited differentiative potential compared with stem cells. Therefore, stem cells and micrografts cannot be considered equivalent, although in some cases they exhibit similar regenerative potential, which is the focus of this review. Last, stem cell therapies remain limited because of complex and costly processes, making them not very feasible in clinical practice, whereas obtaining micrografts is generally a one-step procedure that does not require any advanced tissue manipulation.
Keywords: Mesenchymal stem cell, micrografts, tissue regeneration, autologous, wound management, ulcer
Introduction
The correct histological structure of a given tissue plays a pivotal role in allowing appropriate function and optimizing the activities to which that tissue is predisposed. Unfortunately, many factors can alter the physiology of a tissue. Dermal tissue is the first line of defense of the human body and it is exposed to many risks; therefore, acute or chronic lesions of the skin greatly affect the quality of life of the patient. Tissue repair and regeneration represent a major challenge for the scientific community, which is continually seeking new strategies able to promote this process, especially for tissues in which the regenerative potential is very low, such as heart, nerve, and cartilage.1–3
The percentage success for regenerative medicine is closely related to the biological sources used, such as stem cells, scaffolds, growth factors, and grafts. The main role of regenerative medicine is to replace damaged tissue while maintaining its original function or, alternatively, to stimulate regeneration of the tissue itself, respecting the original histological hierarchy.
The aim of stem cell therapy is to replace a damaged or aged tissue, restoring healthy, functioning cells. In practice, stem cell therapies are based mainly on the use of mesenchymal stem cells (MSCs), which are multipotent cells with unique biological properties.4,5 Several in vitro and preclinical studies have reported that MSCs might be promising in cell therapy because of their ability to differentiate into several cell types and secrete bioactive molecules capable of stimulating recovery of injured cells through a paracrine effect of inflammation inhibition. MSCs also show a lack of immunogenicity and can exert immunomodulatory functions.6 The use of MSCs has been evaluated under different conditions, such as ischemic cardiovascular diseases, critical limb ischemia, bone and cartilage regeneration, and neural diseases.7
Tissue engineering is an emerging interdisciplinary field based on the combined use of scaffolds and biologic mediators such as stem cells and growth factors, providing a new tool for regenerative medicine. An example of tissue engineering is represented by bioengineered dermal substitutes, which not only repair wounds, but also supply growth factors, antibiotics, and anti-inflammatory drugs, which help hasten the wound healing process.8
Another approach is skin grafting, the success of which depends on the engraftment rate into the recipient site. Skin grafting is the most ancient healing technique used worldwide by surgeons to close wounds and, although less ideal than flap closures, can produce a good aesthetic result.9 Although this approach offers a rapid and temporary solution, it can be extremely expensive, especially when the defects are wide, because the availability of donor tissue is always limited.
A valid alternative to the classical skin graft is the micrografting technique, which allows a large defect to be covered using a minimal amount of donor skin by expanding it. However, although micrografting is an appealing strategy for wound coverage, additional studies are needed to identify its true potential and pitfalls.10
Stem cells and micrografts cannot be considered equivalent entities; however, in some cases, they exhibit similar regenerative potential even though some differences exist. Stem cells are self-renewing pluripotent cells with the capacity to differentiate into all cell types; in contrast, micrografts derive from a small fragment of an autologous tissue and exhibit limited differentiative potential compared with stem cells. Recent scientific evidence has shown that micrografts derived by mechanical tissue disaggregation with a cut-off of 80 µm (largest aggregates) are enriched in progenitor cells expressing MSC-like markers and have strong regenerative potential.11 A progenitor cell, like a stem cell, can differentiate into a specific cell type but its differentiation is more specific and committed to its “target” cell. The most important difference between stem cells and progenitor cells is that stem cells can replicate indefinitely, whereas progenitor cells can divide only a limited number of times.12
In the following sections, we will elucidate the main applications, benefits, and limitations of stem cells and micrografts.
Stem cells: definitions, therapy, and limitations
The concept of stem cells was introduced at the end of the last century to account for the regenerative capabilities showed by some tissues. “Stemness” is a defining characteristic of cells able to differentiate into more specialized cells because of pluripotency and self-renewal properties. Pluripotency is the ability to differentiate into all three germ layers: endoderm, mesoderm, and ectoderm,13 whereas self-renewal is the capability of unlimited cell replications while maintaining the same differentiative stages.14 Therefore, stem cells can duplicate practically limitlessly and, to different extents, differentiate into various lineages, making their use appealing when the aim is regenerating damaged tissue in complex injuries.5,15–17 The clinical significance of these populations of stem cells led to 30 years of research in which the greatest challenge was identifying human stem cells and determining how to isolate them. Today, stem cells are classified as embryonic stem cells, adult stem cells, MSCs, and, finally, induced pluripotent stem cells.18 A significant milestone was the pioneering work by Owen and Friedenstein, who first described a population of clonogenic, non-hematopoietic stem cells that were able to differentiate into several lineages, including cartilage, bone, and adipocytes.19 Because of their multipotency, they were named mesenchymal stem cells.20 Contradictory findings about their origins, development, and biological function have prompted the scientific community to better investigate the role of MSCs; however, despite the inadequate understanding of MSCs, ongoing efforts are being made to use these cells in a clinical setting.21
MSCs are applied in different fields of medicine, including orthopedics, wound healing, neurology, oncology, and many others. MSCs are the most investigated stem cell population because of their biological and immunomodulatory properties in addition to ease with which they can be collected from many tissues. A recent review summarized MSC-based clinical trials, either complete or ongoing in the database of the United States National Institutes of Health, including trials for hematological disease, graft-versus-host disease (GvHD), organ transplantation, diabetes, and other diseases involving liver, kidney, lung, cardiovascular system, bone, cartilage, neurological, and autoimmune. The authors of these studies concluded that, in most cases, MSC therapy was efficient and promising in terms of safety, indicating that MSC infusion and administration are well tolerated.7 However, the potential risk of MSC transplantation should be considered based on long-term observations. Other studies focused on MSC paracrine properties, including the release of extracellular vesicles containing mRNAs and regulatory micro-RNAs and secretion of a large number of regulatory substances.22,23 However, if the paracrine effect of MSCs represents a risk, it can also offer an opportunity to develop new therapeutic approaches combining nanomedicine and stem cell therapy to create a novel class of next-generation drug delivery systems. By far, the most widely used source of MSCs is adult bone marrow followed by adipose tissue and, in rare cases, discarded tissues, such as umbilical cord and placental cells.24 In this regard, human adult adipose tissue may be a suitable alternative source of MSCs because adipose stem cells can be largely extracted from subcutaneous adipose tissue and used in surgical practice for tissue remodeling.25,26 In fact, adipose-derived stem cells can undergo multi-lineage differentiation and secrete growth factors that enhance wound-healing processes by promoting angiogenesis, and hence increase local blood supply.27
In recent years, the first stem cell-based therapy—named Holoclar—received market authorization by the European Medicines Agency (EMA).28 This therapy can efficiently replace damaged corneal epithelial cells and is based on the use of ocular limbal cultured stem cells. The most important characteristic of a new therapy for wound healing or epithelial graft is the maintenance of an adequate number of stem cells or progenitors to ensure engraftment of the injured area and increase the success rate of treatment.29
Despite these efforts, very few of the 900 clinical trials officially registered have reached clinical significance (https: //clinicaltrials.gov/); among 19 reported industry-sponsored phase III clinical trials, only 10 were completed and even fewer had publicly available data. Even though those clinical studies using “mesenchymal stem cells” or “medicinal signaling cells” are indicated as safe, results for the majority of listed trials are unavailable.
Among the completed studies, three have been disclosed allowing for public consideration: allogenic marrow MSCs for GvHD, autologous marrow MSCs for heart diseases, and allogenic adipose MSCs for Crohn’s fistula diseases.27 Osiris Therapeutics (Columbia, MD, USA) received Health Canada approval via a Notice of Compliance with Conditions for Prochymal for the treatment of children with refractory GvHD. Prochymal is a cryobanked product containing marrow-derived MSCs sourced from healthy volunteers. Subset analysis showed that children with GvHD were responsive to MSCs.30
MSCs were the first approved allogeneic regenerative medicine product in Japan. In 2003, JCR Pharmaceuticals (Hyogo, Japan), using the licensed technology of Osiris Therapeutics, developed TEMCELL for acute GvHD, which was approved in 2015 by the Japanese Ministry of Labour. The first unambiguously successful use of MSCs resulted from the TiGenix-sponsored trial, NCT01541579. It demonstrated that allogeneic stem cells were significantly and substantially superior to placebo in treating perianal fistulas associated with Crohn’s disease. In that trial, MSCs were retrieved from cryo storage and cultured for a few days before being administered to patients.31
The reason for major concern is not the contradictory research on stem cells, that has led to very few market approvals, but the increasing number of unregistered treatments largely in private clinics around the world that lack approval from regulatory bodies. There is an urgent need to educate patients, doctors, and researchers about MSCs and what the therapeutic opportunities truly are.32
From a regulatory perspective, any treatment or procedure that involves manipulation of stem cells is classified as an advanced therapy medicinal product, which is subject to strict criteria from the regulatory agencies.33 In the last 20 years, the scientific community has experienced exponential growth in clinical trials involving such cells, but unfortunately the scientific rationale and clinical efficacy are unclear, highlighting the need for a different approach to hasten their translational application. Cells should be described not only based on their origin tissue but primarily on their differentiation capacity, as established by rigorous assays.
In summary, despite advances in stem cell therapy, very few clinical results have been achieved until recently. Large-scale clinical trials are necessary to investigate the safety of stem cells, especially immediately and years after transplantation.
Micrografting: theory and applications
The micrografting technique was conceived by Cicero Parker Meek at the University of South Carolina Aiken in 1958.34 Micrografting is based on the concept that by increasing the superficial area of a skin graft by cutting the graft into smaller “micrografts,” it is possible to cover a wound larger than the original donor site. Micrografting was first applied to the treatment of burns because of a lack of available donor sites for skin grafting. The original Meek technique was complex because the micrografts were placed with the dermal side down to achieve optimal survival. Because this requirement of dermal orientation was impracticable, especially with very small graft fragments, the technique did not gain widespread clinical application, and it was eclipsed in 1964 after the introduction of mesh skin grafts by Tanner et al.35 Subsequently, several modifications were made to overcome the limitations; in 1993, Kreis et al. modified Meek’s technique by using a dermatome running on compressed air to obtain widely expanded postage stamp autografts. This modification, combined with the use of cultured grafts or allografts, improved the treatment of severe burns, reaching a percentage of wound coverage of 75%.36 A recent literature review of Meek and mesh techniques indicated that micrografting can be used in burns of >30% total body surface area (TBSA), even with inadequate donor sites and in patients with comorbidities, such as in patients with diabetes with very low vascularity and low metabolic demand.37 To date, the Meek micrografting technique is often used for extensive burn management, and the micrografting concept is strongly related to this context.38,39
Micrografting has subsequently been applied in other clinical settings, such as hair transplantation, hair restoration of the scalp, and regeneration of the face after burn injuries, particularly eyebrow reconstruction.40,41 The micrografting approach can be also used for treatment of vitiligo by melanocyte transplant or autologous punch grafting, resulting in rapid and satisfactory pigmentation.42,43
In recent years, a new clinical approach has been developed related to the micrografting concept, called “Rigenera micrografting technology” (Human Brain Wave LLC, Turin, Italy). The objective of this technology is to mechanically disaggregate autologous tissue, with a calibrated size of 80 µm, collecting autologous micrografts enriched in progenitor cells, growth factors, and particles of extracellular matrix derived from the patient’s own tissue. This technology was developed after several years of experimental and clinical research on stem cell isolation from dental pulp44 to obtain highly viable calibrated micrografts that express MSC markers. In vitro studies have shown that micrografts obtained mechanically by selecting particles with a cut-off of 80 µm are positive for MSC markers such as CD73, CD90, CD115, and CD146 and negative for hematopoietic markers such as CD34 and CD4514,45–47 only. Additionally, these studies have reported cell viability ranging from 70% to 90%. These data support the regenerative potential of micrografts, and several studies have reported the ability of micrografts to differentiate into chondrocytes, osteocytes, and adipocytes.47,48
In vitro studies have shown that micrografts exhibit a fibroblast-like morphology when cultured and have confirmed the expression of MSC markers. When combined with collagen sponges, micrografts can form a viable and proliferative bio-complex, enhancing their regenerative potential.49
Clinically, these micrografts have been applied in different fields of medicine such as dentistry, dermatology, orthopedics, and especially wound care. The first clinical application of autologous micrografts was reported in the dentistry field, showing that micrografts derived from human dental pulp or periosteum promote periodontal regeneration50 and bone regeneration in the atrophic maxilla51 and can preserve the alveolar socket after tooth extraction by reducing bone resorption and increasing new bone formation.52 Furthermore, a recent study reported the ability of autologous micrografts to promote sinus lift augmentation.53
In contrast, the first application of micrografts in wound care was reported 5 years ago, in which the authors described two clinical cases of complex postoperative wounds. In the first case, after abdominoplastic surgery, a woman developed necrosis at the end of the wound flaps and was initially treated with vacuum-assisted closure (VAC) therapy without any improvement of wound margins with respect to skin surface. The application of autologous skin micrografts showed a gradual improvement of the wound’s margins with leveling to the skin surface.54 In the second case, a man underwent numerous different surgical interventions due to the presence of adhesions on the colon, ascites, an entero-cutaneous fistula, and other complications. He was first treated with VAC therapy and then with autologous micrografts, resulting in progressive improvement of wound surface and size reduction.54
One advantage of a micrograft suspension obtained by mechanical disaggregation is that the suspension can be used alone or in combination with different scaffolds or biomaterials to form a bio-complex. Micrografts have been combined with a collagen sponge to treat a post-traumatic lesion previously treated with two radical debridements and negative pressure therapy; the authors reported improvements of both the re-epithelialization process and softness of the lesion.46
Micrografts obtained by mechanical disaggregation of autologous tissue have also been widely used in the management of post-surgical dehiscence in a set of patients. One case report described an oncological and immunocompromised patient who underwent decompressive spinal laminectomy and vertebral fixation, after which a dehiscence occurred. The patient was treated with advanced dressings without any benefit and started a cycle of chemotherapy. Subsequently, the patient was treated with negative wound pressure therapy for 2 months, which resulted in a reduction in diameter and depth of the wound dehiscence but not complete re-epithelialization. After application of micrografts, complete re-epithelialization was achieved within 70 days despite the patient’s comorbidities.55 A similar result on wound dehiscence was achieved in another case that showed good remission of the wound within 1 month, on average, of micrograft application and a complete re-epithelialization after 1 year.56
Chronic wounds represent a major challenge for wound care specialists in terms of management and time, as well as representing an economic burden for the healthcare system.57 Among chronic wounds, non-healing ulcers, especially venous ulcers in the lower extremity, occur very frequently; these wounds reduce the quality of life of affected patients and represent a social issue due to reduced mobility and social isolation. The treatment of chronic venous ulcer includes several approaches, such as compression therapy and debridement when necessary, even if these ulcers do not completely heal in some cases, even after several months.58
The role of micrografts in the treatment of ulcers has been demonstrated in several studies in which micrografts promoted healing of chronic leg ulcers of different etiologies, including venous, diabetic, and post-traumatic ulcers. In the study of Trovato et al., the authors reported the appearance of granulation tissue 3 weeks after micrograft application and the formation of new tissue covering the area around the lesion after 4 weeks. The authors also reported no signs of inflammation in the skin around the wound and a reduction of pain after micrograft application.59 Similar results were observed in another independent study in which the authors evaluated the ability of dermal micrografts to improve the healing of venous, diabetic, pressure, and post-traumatic ulcers. Reductions in wound size, increased granulation, and reduced exudation were reported.49 Another study reported that 15 patients showed wound reduction of 37.33 ±19.35% after 2 weeks, 9 patients were healed after 8 weeks, and 13 patients were healed 16 weeks following micrografting. The quality of scars was good, and they did not deteriorate at the 6-month follow-up.60 In a recent study, the Rigenera micrografting technique was used in 70 cases of traumatic wounds of the lower and upper limbs; in 69 cases, complete healing of the wounds was achieved in a period between 35 and 84 days. This clinical trial was registered at https://clinicaltrials.gov/ct2/show/NCT04030832. On day 0, the mean surface area of the wounds was 14 cm2; on day 14, the mean surface area was 9.3 ±1.6 cm2, representing a reduction of 33.6% ± 11.4%. In the same clinical trial, patients using a visual analog scale to self-assess pain, used the Vancouver Scar Scale to evaluate the aesthetic characteristics of the lesions, and the Wound Bed Score to track to progress of the wound; all scored significant results with optimum outcomes.61
Micrografts have also been used for other non-healing wounds, such as those developing following Fournier’s gangrene or chronic osteomyelitis, showing a reduction in wounds after approximately 1 month of treatment.62,63 The efficacy of micrografts has also been reported in repairing cartilage defects, for cartilage regeneration in patients affected by external nasal valve collapse,64 and the treatment of function-limiting and painful knee chondral injuries.65 An in vitro study showed that autologous micrografts influenced chondrocyte differentiation, increasing deposition of glycosaminoglycans and the presence of collagen II in primary human cells cultured in the presence of micrografts, which supported the formation of chondrogenic micro-masses and acted as a scaffold for chondrocytes.66
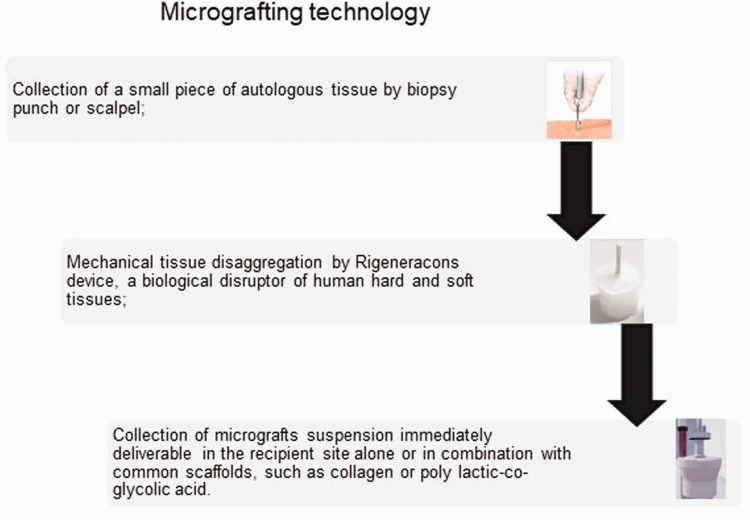
Finally, a recent clinical trial reported good outcomes in terms of safety and feasibility using autologous micrografts derived by atrial appendages during on-pump coronary artery bypass graft surgery for epicardial transplantation.67 Figure 1 shows the different steps to obtain autologous micrografts by mechanical disaggregation. From a biological point of view, a recent in vivo and in vitro study showed that micrografts improve skin re-epithelialization by accelerating the migration of fibroblasts and keratinocytes by activation of the extracellular signal-regulated kinase (ERK) signaling pathway. Specifically, micrograft-treated wounds showed an improvement in granulation tissue formation, organized collagen content, and newly formed blood vessels.68
Figure 1.
Representative diagram of micrografting technology.
In summary, good clinical evidence supports the role of autologous micrografts in different medical settings of tissue repair and regeneration, such as bone and cartilage regeneration, cardiac applications, and wound care management.
Conclusions
Despite recent progress in stem cell therapy, few clinically significant results have been reported. The clinical applications of stem cell therapy are reduced by the complexity of the technique and the enormous expense, making this approach time consuming and not very feasible. There is a growing need for more practical and reasonable approaches, especially for diseases that are not life threatening. In this context, micrografts represent an innovative tool for clinicians because of their ability to stimulate tissue regeneration. Additionally, from a regulatory perspective, micrografts are considered a normal graft and do not require extensive manipulation before application, resulting in a greater safety profile.
Declaration of conflicting interest
All authors belong to the Scientific Division of Human Brain Wave SRL (Turin, Italy), the owner company of the Rigenera technology cited in this review and developed to obtain autologous micrografts.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Carlo Astarita https://orcid.org/0000-0003-3010-9878
Letizia Trovato https://orcid.org/0000-0001-6004-795X
References
- 1.Gorabi AM, Tafti SHA, Soleimani M, et al. Cells, scaffolds and their interactions in myocardial tissue regeneration. J Cell Biochem 2017; 118: 2454–2462. doi: 10.1002/jcb.25912. [DOI] [PubMed] [Google Scholar]
- 2.Zhang BG, Quigley AF, Myers DE, et al. Recent advances in nerve tissue engineering. Int J Artif Organs 2014; 37: 277–291. doi: 10.5301/ijao.5000317. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone B, Alini M, Cucchiarini M, et al. Tissue engineering for articular cartilage repair-the state of the art. Eur Cell Mater 2013; 25: 248–267. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI. Adult mesenchymal stem cells: when, where, and how. Stem Cells Int 2015; 2015: 628767. doi: 10.1155/2015/628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown C, McKee C, Bakshi S, et al. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med 2019; 13: 1738–1755. doi: 10.1002/term.2914 [DOI] [PubMed] [Google Scholar]
- 6.Naji A, Eitoku M, Favier B, et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci 2019; 76: 3323–3348. doi: 10.1007/s00018-019-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant 2016; 25: 829–848. [DOI] [PubMed] [Google Scholar]
- 8.Yu JR, Navarro J, Coburn JC, et al. Current and future perspectives on skin tissue engineering: key features of biomedical research, translational assessment, and clinical application. Adv Healthc Mater 2019; 8: e1801471. doi: 10.1002/adhm.201801471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prohaska J, Cook C. Skin grafting. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2019. [Google Scholar]
- 10.Biswas A, Bharara M, Hurst C, et al. The micrograft concept for wound healing: strategies and applications. J Diabetes Sci Technol 2010; 4: 808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trovato L, Monti M, Del Fante C, et al. A new medical device rigeneracons allows to obtain viable micro-grafts from mechanical disaggregation of human tissues. J Cell Physiol 2015; 230: 2299–2303. [DOI] [PubMed] [Google Scholar]
- 12.Seaberg RM, Van Der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci 2003; 26: 125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 13.Byrne J. The definition and etymology of the word pluripotency. eJournal of Cellular Biotechnology 2011; 1: eP2. [Google Scholar]
- 14.Becker AJ, Mcculloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 1963; 197: 452–454. [DOI] [PubMed] [Google Scholar]
- 15.Mazini L, Rochette L, Amine M, et al. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci 2019; 20: pii: E2523. doi: 10.3390/ijms20102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhat M, Shetty P, Shetty S, et al. Stem cells and their application in dentistry: a review. J Pharm Bioallied Sci 2019; 11: S82–S84. doi: 10.4103/JPBS.JPBS_288_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu DT, Nguyen Thi Phuong T, Tien NLB, et al. Adipose tissue stem cells for therapy: an update on the progress of isolation, culture, storage, and clinical application. J Clin Med 2019; 8: pii: E917. doi: 10.3390/jcm8070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep 2015; 35: pii: e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 1988; 136: 42–60. [DOI] [PubMed] [Google Scholar]
- 20.Caplan AI. Mesenchymal stem cells. J Orthop Res 1991; 9: 641–650. [DOI] [PubMed] [Google Scholar]
- 21.Martin I, Galipeau J, Kessler C, et al. Challenges for mesenchymal stromal cell therapies. Sci Transl Med 2019; 11: pii: eaat2189. doi: 10.1126/scitranslmed.aat2189. [DOI] [PubMed] [Google Scholar]
- 22.Crivelli B, Chlapanidas T, Perteghella S, et al. Mesenchymal stem/stromal cell extracellular vesicles: from active principle to next generation drug delivery system. J Control Release 2017; 262: 104–117. [DOI] [PubMed] [Google Scholar]
- 23.Park WS, Ahn SY, Sung SI, et al. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr Res 2018; 83: 214–222. [DOI] [PubMed] [Google Scholar]
- 24.Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 2018; 22: 824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Francesco F, Ricci G, D’Andrea F, et al. Human adipose stem cells: from bench to bedside. Tissue Eng Part B Rev 2015; 21: 572–584. doi: 10.1089/ten.TEB.2014.0608. [DOI] [PubMed] [Google Scholar]
- 26.Nicoletti GF, De Francesco F, D’Andrea F, et al. Methods and procedures in adipose stem cells: state of the art and perspective for translation medicine J Cell Physiol 2015; 230: 489–495. doi: 10.1002/jcp.24837. [DOI] [PubMed] [Google Scholar]
- 27.Bertozzi N1, Simonacci F, Grieco MP, et al. The biological and clinical basis for the use of adipose-derived stem cells in the field of wound healing. Ann Med Surg (Lond) 2017; 20: 41–48. doi: 10.1016/j.amsu.2017.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrini G, Ardigò D, Milazzo G, et al. Navigating market authorization: the path Holoclar took to become the first stem cell product approved in the European Union. Stem Cells Transl Med 2018; 7: 146–154. doi: 10.1002/sctm.17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Luca M, Aiuti A, Cossu G, et al. Advances in stem cell research and therapeutic development. Nat Cell Biol 2019; 21: 801–811. doi: 10.1038/s41556-019-0344-z. [DOI] [PubMed] [Google Scholar]
- 30.Kurtzberg J, Prockop S, Teira P, et al. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant 2014; 20: 229–235. doi: 10.1016/j.bbmt.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 31.Panes J. Stem cell therapy for perianal fistulas in Crohn’s disease. Gastroenterol Hepatol (NY) 2016; 12: 637–640. [PMC free article] [PubMed] [Google Scholar]
- 32.Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature 2018; 561: 455–457. doi: 10.1038/d41586-018-06756-9. [DOI] [PubMed] [Google Scholar]
- 33.Detela G, Lodge A. EU regulatory pathways for ATMPs: standard, accelerated and adaptive pathways to marketing authorisation. Mol Ther Methods Clin Dev 2019; 13: 205–232. doi: 10.1016/j.omtm.2019.01.010. eCollection 2019 Jun 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meek CP. Successful microdermagrafting using the Meek-Wall microdermatome. Am J Surg 1958; 96: 557. [DOI] [PubMed] [Google Scholar]
- 35.Tanner JC, Jr, Vandeput J, Olley JF. The mesh skin graft. Plast Reconstr Surg 1964; 34: 287–292. [PubMed] [Google Scholar]
- 36.Kreis RW, Mackie DP, Vloemans AW, et al. Widely expanded postage stamp skin grafts using a modified Meek technique in combination with an allograft overlay. Burns 1993; 19: 142–145. [DOI] [PubMed] [Google Scholar]
- 37.Quintero EC, Escrivá Machado JF, Damian Robles RA. Meek micrografting history, indications, technique, physiology and experience: a review article. J Wound Care 2018; 27: S12–S18. DOI: 10.12968/jowc.2018.27.Sup2.S12 [DOI] [PubMed] [Google Scholar]
- 38.Klosová H, Němečková Crkvenjaš Z, Štětinský J. . Meek micrografting technique and its use in the treatment of severe burn injuries at the University Hospital Ostrava burn center. Acta Chir Plast 2017; 59: 11–17. [PubMed] [Google Scholar]
- 39.Houschyar KS, Tapking C, Nietzschmann I, et al. Five years experience with meek grafting in the management of extensive burns in an adult burn center. Plast Surg (Oakv) 2019; 27: 44–48. doi: 10.1177/2292550318800331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrera A. The use of micrografts and minigrafts for the treatment of burn alopecia. Plast Reconstr Surg 1999; 103: 581–584. [DOI] [PubMed] [Google Scholar]
- 41.Motamed S, Davami B. Eyebrow reconstruction following burn injury. Burns 2005; 31: 495–499. [DOI] [PubMed] [Google Scholar]
- 42.Sharquie KE, Noaimi AA, Al-Mudaris HA. Melanocytes transplantation in patients with vitiligo using needling micrografting technique. J Drugs Dermatol 2013; 12: e74–e78. [PubMed] [Google Scholar]
- 43.Saldanha KD, Machado Filho CD, Paschoal FM. Action of topical mometasone on the pigmented halos of micrografting in patients with vitiligo. An Bras Dermatol 2012; 87: 685–690. [DOI] [PubMed] [Google Scholar]
- 44.D’Aquino R, De Rosa A, Lanza V, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cells and Mater 2009; 18: 73–85. [DOI] [PubMed] [Google Scholar]
- 45.Zanzottera F, Lavezzari E, Trovato L, et al. Adipose derived stem cells and growth factors applied on hair transplantation. Follow-up of clinical outcome. J Cosmet Dermatol Sci Appl 2014; 24: 268–274. DOI: 10.4236/jcdsa.2014.44036 [Google Scholar]
- 46.Purpura V, Bondioli E, Graziano A, et al. Tissue characterization after a new disaggregation method for skin micro-grafts generation. J Vis Exp 2016; 109: e53579. doi: 10.3791/53579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monti M, Graziano A, Rizzo S, et al. In vitro and in vivo differentiation of progenitor stem cells obtained after mechanical digestion of human dental pulp. J Cell Physiol 2017; 232: 548–555. doi: 10.1002/jcp.25452 [DOI] [PubMed] [Google Scholar]
- 48.Senesi L, De Francesco F, Farinelli L, et al. Mechanical and enzymatic procedures to isolate the stromal vascular fraction from adipose tissue: preliminary results. Front Cell Dev Biol 2019; 7: 88. doi: 10.3389/fcell.2019.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Francesco F, Graziano A, Trovato L, et al. A regenerative approach with dermal micrografts in the treatment of chronic ulcers. Stem Cell Rev 2017; 13: 149. doi: 10.1007/s12015-016-9698-9 [DOI] [PubMed] [Google Scholar]
- 50.Graziano A, Carinci F, Scolaro S, et al. Periodontal tissue generation using autologous dental ligament micro-grafts: case report with 6 months follow-up. Ann Oral Max Surg 2013; 1: 20. [Google Scholar]
- 51.Brunelli G, Motroni A, Graziano A, et al. Sinus lift tissue engineering using autologous pulp micro-grafts: a case report of bone density evaluation. J Indian Soc Periodontol 2013; 17: 644–667. doi: 10.4103/0972-124X.119284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Aquino R, Trovato L, Graziano A, et al. Periosteum-derived micro-grafts for tissue regeneration of human maxillary bone. J Transl Sci 2016; 2: 125–129. doi: 10.15761/JTS.1000128 [Google Scholar]
- 53.Rodriguez Y, Baena R, D’Aquino R, et al. Autologous periosteum-derived micrografts and PLGA/HA enhance the bone formation in sinus lift augmentation. Front Cell Dev Biol 2017; 5: 87. doi: 10.3389/fcell.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giaccone M, Brunetti M, Camandona M, et al. A new medical device, based on Rigenera protocol, in the management of complex wounds. J Stem Cells Res Rev & Rep 2014; 1: 1013. [Google Scholar]
- 55.Baglioni E, Trovato L, Marcarelli M, et al. Treatment of oncological post-surgical wound dehiscence with autologous skin micrografts. Anticancer Res 2016; 36: 975–980. [PubMed] [Google Scholar]
- 56.Marcarelli M, Trovato L, Novarese E, et al. Rigenera protocol in the treatment of surgical wound dehiscence. Int Wound J 2017; 14: 277–281. doi: 10.1111/iwj.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen 2019; 27: 114–125. doi: 10.1111/wrr.12683 [DOI] [PubMed] [Google Scholar]
- 58.Xie T, Ye J, Rerkasem K, et al. The venous ulcer continues to be a clinical challenge: an update. Burns Trauma 2018; 6: 18. doi: 10.1186/s41038-018-0119-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trovato L, Failla G, Serantoni S, et al. Regenerative surgery in the management of the leg ulcers. J Cell Sci Ther 2016; 7: 238. doi: 10.4172/2157-7013.1000238 [Google Scholar]
- 60.Miranda R, Farina E, Farina MA. Micrografting chronic lower extremity ulcers with mechanically disaggregated skin using a micrograft preparation system. J Wound Care 2018; 27: 60–65. [DOI] [PubMed] [Google Scholar]
- 61.Riccio M, Marchesini A, Zingaretti N, et al. A multicentre study: the use of micrografts in the reconstruction of full-thickness post-traumatic skin defects of the limbs – A whole innovative concept in regenerative surgery. Stem Cell Int 2019; 2019: Article ID 5043518. DOI: 10.1155/2019/5043518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bocchiotti MA, Bogetti P, Parisi A, et al. Management of Fournier’s gangrene non-healing wounds by autologous skin micrograft biotechnology: a new technique. J Wound Care 2017; 26: 314–317. doi: 10.12968/jowc.2017.26.6.314 [DOI] [PubMed] [Google Scholar]
- 63.Uehara M, Shimizu F. Progress report for intractable ulcer and osteomyelitis cases using autologous micrografts. SAGE Open Med Case Rep 2019; 7: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ceccarelli G, Gentile P, Marcarelli M, et al. In vitro and in vivo studies of alar-nasal cartilage using autologous micro-grafts: the use of the Rigenera® protocol in the treatment of an osteochondral lesion of the nose. Pharmaceuticals (Basel) 2017; 10: pii: E53. doi: 10.3390/ph10020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dorta Fernandez A, Baroni Luengo A. . Biostimulation of knee cartilage using autologous micro-grafts: a preliminary study of the Rigenera protocol in osteochondral lesions of the knee. Rehabil Sci 2018; 3: 8–12. [Google Scholar]
- 66.Viganò M, Tessaro I, Trovato L, et al. Rationale and pre-clinical evidences for the use of autologous cartilage micrografts in cartilage repair. J Orthop Surg Res 2018; 13: 279. doi: 10.1186/s13018-018-0983-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nummi A, Nieminen T, Pätilä T, et al. Epicardial delivery of autologous atrial appendage micrografts during coronary artery bypass surgery-safety and feasibility study. Pilot Feasibility Stud 2017; 3: 74. doi: 10.1186/s40814-017-0217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balli M, Vitali F, Janiszewski A, et al. Autologous micrograft accelerates endogenous wound healing response through ERK-induced cell migration. Cell Death Differ 2019. doi: 10.1038/s41418-019-0433-3 [DOI] [PMC free article] [PubMed]