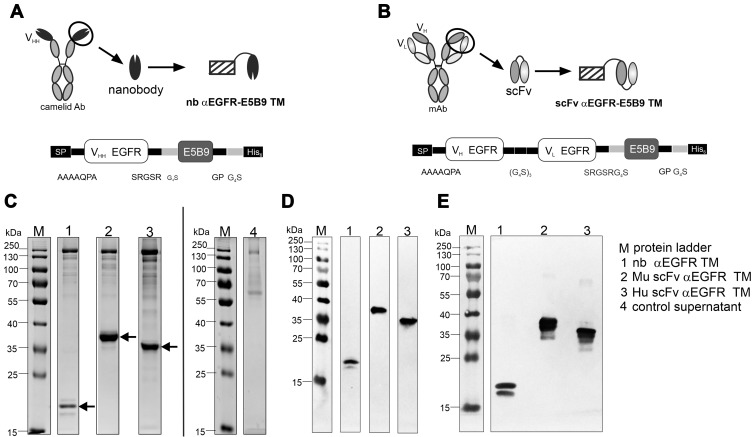
Figure 2.
Construction and biochemical characterization of αEGFR TMs. The pre-existing nanobody (nb) αEGFR target module (TM) consists of one single camelid antibody-derived (clone 7C12) domain to enable EGFR binding and the E5B9 tag for UniCAR recognition (A). Novel murine (Mu) and humanized (Hu) αEGFR TMs consist of the single-chain fragment variable (scFv) of the αEGFR mAb Cetuximab and the E5B9 tag (B). The recombinant TMs were further equipped with 6xHis residues (His6) for protein purification and detection (A, B). Amino acids of linker elements are indicated (A, B). Recombinant TMs were expressed by 3T3 cells after transduction. Eluted and dialyzed fractions were separated via SDS-PAGE and stained with Coomassie brilliant blue G-250 (C) or detected after immunoblotting via αHis mAb (D) or αE5B9 mAb (E) on a nitrocellulose membrane. Arrows indicate the recombinant TMs. Supernatant of non-transduced 3T3 wild type cells was analyzed as a negative control (control supernatant) in the Coomassie-stained gel (C). (VH, variable heavy chain domain; VL, variable light chain domain).