Abstract
Quitting smoking among people living with HIV/AIDS (PLWHA) is a priority. aHowever, PLWHA and clinicians working with this population are reluctant to use tobacco use treatments out of concern that smoking cessation can diminish anti-retroviral therapy (ART) adherence and quality of life (QoL) and increase psychiatric symptoms. This secondary analysis from a placebo-controlled varenicline trial for tobacco dependence among PLWHA (N=179) examined if smoking cessation at the end of treatment (EOT) was associated with changes in ART adherence, QoL, anxiety and depression symptoms, and varenicline side effects. ART adherence was not affected by smoking cessation (p>0.05), remaining ≥98% for all participants. Across 8 subscales measuring QoL, 7 remained unchanged over time across smokers and abstainers; side effects were not associated with cessation. Controlling for baseline smoking rate, adherence to varenicline/placebo and counseling, and treatment arm, participants who had quit smoking at the EOT reported a significant reduction in depression (β = −1.657, 95% CI: −2.893, −0.422, p=.009) and anxiety (β = −1.434, 95% CI: −2.812, −0.56, p=.041) and increased life satisfaction (β = 0.88, 95% CI: 0.21, 3.275, p=.027). When PLWHA quit smoking they may not experience adverse clinical outcomes including ART non-adherence and may experience beneficial psychological effects, supporting the use of FDA-approved smoking cessation treatments in this population.
Keywords: Tobacco, HIV, Smoking, Varenicline, Quality of Life, Outcomes
Introduction
The advent of anti-retroviral therapy (ART) for people living with HIV/AIDS (PLWHA) has not only substantially improved life expectancy (Wandeler, Johnson, & Egger, 2016), but it has also led to the critical need to address modifiable risk factors associated with cancer and cardiovascular disease, such as tobacco smoking (Althoff, 2016; Petoumenos & Law, 2016). HIV-infected smokers on antiretroviral therapy lose more life-years due to tobacco use than they do to their HIV infection (Helleberg et al., 2012). On a US population level, tobacco cessation among PLWHA could save 265,000 life-years and would yield greater individual life-years saved than either hepatitis C treatment or ART for those with higher CD4+ counts (Reddy et al., 2016). Unfortunately, the prevalence of tobacco use among PLWHA in the US is about 40% (Raposeiras-Roubin, Abu-Assi, & Iniguez-Romo, 2017), which is close to three times greater than in the general population (Mdodo et al., 2015; Norris, Schiller, & Clarke, 2018). Higher smoking rates among PLWHA, compared to the general population, may be associated with socio-demographic (e.g., low socio-economic status) or psychological (e.g., depressive symptoms) factors or higher nicotine metabolism (Nahvi & Cooperman, 2009; Ashare et al., 2019b).
There are indications that PLWHA who smoke and clinicians working with this population are reluctant to use FDA-approved medications for tobacco dependence (e.g., nicotine replacement therapies, bupropion), particularly varenicline given past studies indicating adverse pstchiatric side effects. For example, about 9% of smokers in the general population report using varenicline (Jarlenski, Baik, & Zhang, 2016), but less than 4% of PLWHA report using varenicline (Pacek, Rass, & Johnson, 2017). Likewise, only 1 in 5 clinicians caring for PLWHA recommend varenicline to their patients who smoke (Pacek, Rass, & Johnson, 2017), and 14% reported prescribing varenicline frequently to patients who smoke (Shuter et al., 2012). The reduced interest among PLWHA in using medications to quit smoking and the reduced willingness to prescribe such medications among clinicians working with this population may stem from concerns about potential adverse clinical effects of quitting smoking, including negative psychiatric reactions resulting in diminished adherence to ART.
Therefore, this study, using data collected from a randomized placebo-controlled clinical trial of varenicline for smokers with HIV, was designed to assess changes in ART adherence, depression and anxiety symptoms, quality of life (QoL), and varenicline side effects over 12 weeks of smoking cessation treatment. These outcomes were measured between PLWHA that quit smoking and those who did not at the end of treatment. Evaluating potential changes in such clinical outcomes may help PLWHA and clinicians working with this population of smokers to make more informed decisions about engaging in smoking cessation treatment, which may help lower the rate of smoking in this population and improve health outcomes.
Methods
For this study, we used data from a completed clinical trial ( NCT01710137) that compared placebo to varenicline for tobacco use among PLWHA. The complete methods and results of this trial, which was approved by the University of Pennsylvania IRB and was conducted between October 2012 and June 2018, have been reported elsewhere (Ashare et al., 2019a).
Participants
Participants were recruited through Penn’s health system, media ads, and through a community-based HIV clinic. Participants were age 18 or older, had a confirmed HIV diagnosis and were treated with ART, and had plasma HIV viral loads <1000 copies/ml. Exclusion criteria included a lifetime history of psychosis or a suicide attempt, self-reported current or planned pregnancy, self-reported current use of smoking cessation medications, and indications of unstable or untreated alcohol/substance abuse; 179 individuals were eligible, willing to enroll, completed the pre-quit session, and were randomized to varenicline (n=89) or placebo (n=90). Participant characteristics for the sample have been reported previously (Ashare et al., 2019a; 2019b).
Interventions
Varenicline was provided at Week 0 based on U.S. Food and Drug Administration labeling: Day 1-Day 3 (0.5 mg once daily); Day 4–7 (0.5 mg twice daily); and Day 8-Day 84 (1.0 mg twice daily). Placebo was identical in appearance and dosing regimen. All participants were offered six standardized, Public Health Service guideline-based smoking cessation counseling sessions at Weeks 0, 1, 3, 5, 7, and 9, in-person or by telephone (Fiore et al., 2008). All sessions were one-on-one and delivered by trained counselors who were supervised by a clinical psychologist. Sessions were designed to help participants understand the risks associated with smoking, prepare for a target quit-date at Week 1 and develop skills to manage nicotine withdrawal and avoid relapse (Lerman et al., 2015; Schnoll et al., 2015; Schnoll et al., 2010).
Measures
At a baseline visit (Week 0), demographic (e.g., age, race, education), HIV-related (e.g., mode of transmission, viral load, type of ARTs) and smoking-related (e.g., current smoking rate, salivary cotinine level [ng/mL], and breath carbon monoxide [CO; ppm]) variables were evaluated.
Varenicline/placebo adherence was assessed using the timeline follow-back method (Brown, 1998) and medication counts from blister-packs (Lerman et al., 2015; Crawford et al., 2019). The total pills taken out of the total pills prescribed (i.e., 165) was computed for an overall proportion of medication adherence. Counseling adherence was also assessed as number of sessions completed out of total prescribed.
The Hospital Depression and Anxiety Scale (Zigmond, & Snaith, 1983), a 14-item self-report measure, assessed depression and anxiety symptoms at Week 0 and EOT. The HIV/AIDS-Targeted Quality of Life Scale (Holmes & Shea, 1998) measured overall functioning and QoL subscales of life satisfaction, health worries, HIV mastery, financial worries, disclosure worries, provider trust and sexual functioning at Week 0 and EOT. ART adherence was assessed with the Adult AIDS Clinical Trials Group Adherence to Antiretrovirals Instrument (Chesney et al., 2000) and pharmacy refill data (Grossberg, Zhang, & Gross, 2004). We computed an adherence variable, for Week 0 and EOT, that consisted of the percentage of doses taken over doses prescribed according to medical refill records for the 2 weeks preceding the assessment time-point (Gross et al., 2006). Varenicline-related side effects (e.g., nausea, sleep problems) were rated from 0 (none) to 3 (severe) and ratings were summed and averaged to create a side-effects measure.
Smoking behavior was assessed during treatment using the timeline follow-back procedure (Lerman et al., 2015; Schnoll et al., 2015). The primary smoking cessation outcome was 7-day point-prevalence abstinence at Week 12 (EOT), based on no self-reported tobacco use (not even a puff) during the 7 days preceding the assessment and a CO ≤ 10ppm (Hughes et al., 2003; Benowitz et al., 2002). Participants who completed the pre-quit session (Week 0) and were randomized to a treatment arm but were lost to follow-up or failed to provide the CO measure were considered not abstinent.
Data Analysis
We used ANOVA and chi-square statistics to examine baseline demographic, HIV-related, smoking-related and treatment-related variables as potential correlates of EOT smoking cessation in order to identify covariates for analyses within a multivariate model. We used repeated measures ANOVA to assess the relationship between EOT smoking cessation and changes from Week 0 to EOT in depression and anxiety symptoms, ART adherence, each QoL subscale, and side effects (overall mean and nausea and sleep problems). The time X smoking cessation status at EOT interaction effect was used to determine the relationship between smoking cessation and clinical outcomes (p<0.05). Variables associated with EOT smoking cessation (p<0.05) were included in a multivariate analysis of variance which controlled for covariates and multiple comparisons.
Results
Baseline Covariates of EOT Smoking Cessation
Differences in baseline characteristics between participants smoking or having quit smoking at EOT are shown in Table 1. While no demographic or disease-related variables were associated with EOT smoking cessation, baseline rates of smoking (F[1,175] = 5.33, p=0.022) and cotinine (F[1,127] = 5.48, p=0.021) were both associated with EOT smoking cessation; participants who had quit smoking at EOT reported smoking fewer cigarettes per day at baseline and had lower cotinine levels at baseline, compared to participants who were smoking at EOT. Likewise, treatment arm (χ2[1] = 6.04, p=0.017), varenicline/placebo adherence (F[1,177] = 4.12, p=0.044), and counseling adherence (F[1,177] = 6.84, p=0.01) were associated with EOT smoking; participants who had quit smoking at EOT were more likely to have received varenicline than placebo and showed higher levels of varenicline/placebo and counseling adherence. These variables were controlled for in subsequent analyses.
Table 1.
Baseline Characteristic of the sample by EOT Smoking Cessation Status
| Smokers (N=143) | Quitters (N=36) | Total (N=179) | |
|---|---|---|---|
| Variable | N (%) or M (SD) | N (%) or M (SD) | N (%) or M (SD) |
| Demographic variables | |||
| Race (% African American) | 114 (79.7) | 32 (88.9) | 146 (81.6) |
| Sex (% Male) | 95 (66.4) | 27 (75.0) | 122 (68.2) |
| Education (% High School Grad or less) | 82 (57.3) | 19 (52.8) | 101 (56.4) |
| Annual Household Income (<20K) | 102 (71.3) | 24 (68.6) | 126 (70.8) |
| Age (Range: 21–68) | 48.2 (9.7) | 50.1 (10.4) | 48.6 (9.9) |
| BMI (Range: 15.3–57.9) | 27.3 (5.8) | 27.5 (9.6) | 27.3 (6.7) |
| # Alcohol Drinks in Past 7 Days (Range: 0–15) | 1.6 (3.0) | 2.4 (5.2) | 1.8 (3.6) |
| Smoking-related variables | |||
| FTND (Range: 0–10) | 4.8 (1.9) | 4.4 (2.5) | 4.7 (2.1) |
| Cigarettes/Day in Past 7 Days (Range: 1–60)a | 12.2 (8.3) | 8.8 (5.1) | 11.5 (7.9) |
| Breath CO, ppm (Range: 1–60) | 14.6 (9.2) | 12.0 (9.2) | 14.1 (9.2) |
| Number of Years Smoking (Range: 4–56) | 31.6 (10.8) | 31.0 (11.3) | 31.5 (10.9) |
| Number of Times Quit Smoking for>24 Hours (Range: 0–500) | 7.7 (42.8) | 3.7 (5.3) | 6.9 (38.2) |
| Cotinine, ng/mL (Range: 0.97–634.5) a | 187.1 (128.3) | 121.2 (119.2) | 174.3 (128.7) |
| Disease-related characteristics | |||
| % of ART Prescribed in Past 2 Weeks Taken (Range: 79–100) | 98 (3.9) | 98 (5.0) | 98 (4.0) |
| % Undetectable Viral Load (<50 copies/ml) | 114 (79.7) | 30 (83.3) | 144 (80.4) |
| CD4+ cells/mm3 (Range: 214–1932) | 691.1 (321.7) | 796.4 (333.9) | 712.8 (326.1) |
| % Acquired HIV via Sex | 116 (81.1) | 28 (77.8) | 144 (80.4) |
| % ART regimen containing efavirenz | 23 (16.4) | 9 (26.5) | 32 (18.4) |
| Treatment-related variables | |||
| Treatment Arm (% Varenicline) a | 64 (44.8) | 25 (69.4) | 89 (49.7) |
| Varenicline/Placebo Adherence (0–192) a | 116.4 (59.5) | 141.0 (37.4) | 121.3 (56.6) |
| Counseling Adherence (Range :1–6) a | 5.3 (1.5) | 5.9 (0.2) | 5.4 (1.3) |
Note. BMI=Body Mass Index; CO=Carbon Monoxide; FTND=Fagerstrom Test for Nicotine Dependence
group difference, p<.05
Clinical Outcomes as Correlates of EOT Smoking Cessation
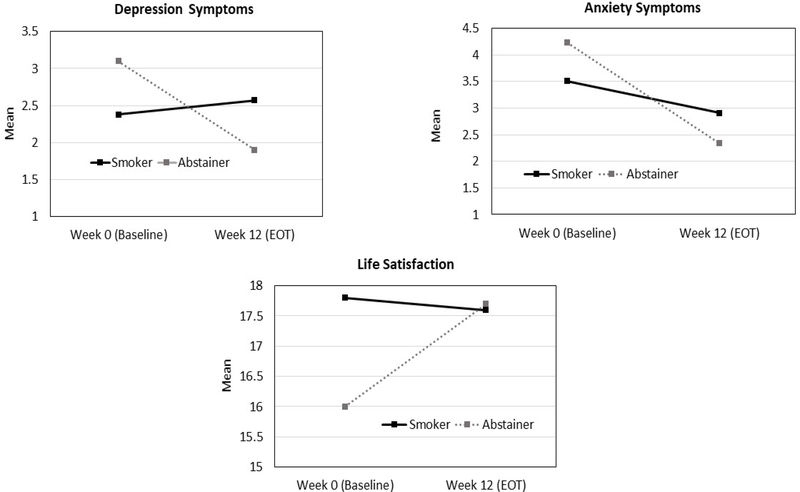
Our models of changes in clinical outcomes over time between EOT smokers and quitters, controlling for covariates, indicated that there were no significant differences over time and between the groups in terms of mean side effects, sleep problems, nausea, ART adherence and the QoL subscales of health worries, HIV mastery, financial worries, disclosure worries, provider trust, and sexual functioning (p’s >0.05). In contast, participants who had quit smoking at EOT reported a significant decrease in depression symptoms, from 3.1 to 1.9, but depression symptoms were stable among smokers (2.4 to 2.6; F[1,97] = 5.75, p=0.02). Likewise, participants who had quit smoking at EOT reported a significantly greater decrease in anxiety symptoms (4.2 to 2.3), compared to those who were smoking at EOT (3.5 to 2.9; F[1,97] = 4.82, p=0.03). Lastly, participants who had quit smoking at EOT reported a significant increase in life satisfaction (16.0 to 17.7; (F[1,96] = 5.1, p=0.03), compared to participants who were smoking at EOT (17.8 to 17.6). These results are shown in Figure 1.
Figure 1.
Changes in Anxiety and Depression Symptoms and Life Satisfaction between Smokers and Abstainers at EOT
Multivariate Model
Variables that were significantly associated with EOT smoking status were entered into a multivariate model (Table 2). Consistent with the univariate analyses, compared to participants smoking at EOT, participants who had quit smoking at the EOT reported a significant reduction in depression symptoms (β = −1.657, 95% CI: −2.893, −0.422, p=.009) and anxiety symptoms (β = −1.434, 95% CI: −2.812, −0.56, p=.041) and increased life satisfaction (β = 0.88, 95% CI: 0.21, 3.275, p=.027).
Table 2.
Multivariate Model of Clinical Outcomes Associated with Smoking Status at EOT, Controlling for Covariates
| β | 95% CI | p | |
|---|---|---|---|
| Depression | |||
| Cigarettes/Day in Past 7 Days | .055 | −.010, .120 | 0.097 |
| Cotinine | .005 | .001, .010 | 0.01 |
| Varenicline/Placebo Adherence | .010 | −.005, .025 | 0.185 |
| Counseling Adherence | .111 | −1.031, 1.253 | 0.847 |
| Treatment Arm | .457 | −.595, 1.508 | 0.391 |
| EOT Smoking Status (Reference = Smoker) | −1.657 | −2.893, −.422 | 0.009 |
| Anxiety | |||
| Cigarettes/Day in Past 7 Days | .025 | −.048, .098 | 0.497 |
| Cotinine | −.002 | −.007, .002 | 0.338 |
| Varenicline/Placebo Adherence | .021 | .004, .038 | 0.018 |
| Counseling Adherence | −1.536 | −2.810, −.263 | 0.019 |
| Treatment Arm | 1.717 | .544, 2.889 | 0.005 |
| EOT Smoking Status (Reference = Smoker) | −1.434 | −2.812, −.056 | 0.041 |
| Life Satisfaction | |||
| Cigarettes/Day in Past 7 Days | −.004 | −.085, .077 | 0.925 |
| Cotinine | −.003 | −.008, .002 | 0.298 |
| Varenicline/Placebo Adherence | −.013 | −.032, .006 | 0.180 |
| Counseling Adherence | 1.540 | .121, 2.958 | 0.034 |
| Treatment Arm | .880 | −.426, 2.186 | 0.184 |
| EOT Smoking Status (Reference = Smoker) | 1.741 | .206, 3.275 | 0.027 |
Discussion
This study sought to assess changes in clinical outcomes following 12 weeks of treatment with varenicline and behavioral smoking cessation counseling between PLWHA who quit smoking and those who had relapsed at the end of 12 weeks. Since PLWHA who smoke and clinicians caring for this population may be concerned that quitting smoking may undermine ART adherence or diminish psychological well-being and QoL, utilization of approved treatments for tobacco use may be low. While this study was exploratory, the results indicate that the differences detected between smokers and those who quit smoking following treatment were opposite of the concerns that PLWHA and clinicians who care for these patients may express when considering treating tobacco dependence.
Indeed, controlling for smoking and treatment characteristics related to smoking cessation outcomes, the present data indicate that any changes for those who quit smoking reflected positive outcomes, including reduced depression and anxiety symptoms and improved life satisfaction. These findings challenge long-standing assumptions that quitting smoking reduces psychological well-being (Aubin 2009) and, instead, is consistent with more recent studies that show improved psychological outcomes following smoking cessation (Rodríguez-Cano et al., 2018; Stepankova et al., 2017; Taylor et al., 2014; Shahab et al., 2014). Importantly, the present results extend these more recent studies from the general population of smokers to a clinical population where concerns for adverse psychological reactions to smoking cessation may be amplified and particularly related to under-utilization of effective smoking cessation treatments.
Further, the present results also show that quitting smoking does not increase the risk for reduced adherence to ART. One may postulate that quitting smoking could infer a sense of health that makes ART adherence less of a priority and that would be an important adverse outcome given the impact of suppressed viral load on life expectancy in this population. However, this does not appear to be the case; adherence to ART remained above 98% across the 12 weeks of treatment for both smokers and quitters. This finding is consistent with recent studies showing >90% adherence among PLWHA who had quit smoking (King et al., 2018) and other studies that have found no relationship between smoking and ART adherence (Moreno, Catley, Lee & Goggin, 2015). Smoking may still be a risk factor for non-adherence to ART (King et al., 2012), but cessation does not appear to undermine ART adherence. Likewise, smoking cessation was not associated with an increase in varenicline-related side effects, including nausea and sleep problems, which have been associated with medication discontinuation in the general population and lower smoking cessation rates (Drovandi et al., 2016).
These results should be considered in the context of study limitations. First, this study was exploratory in nature and was not meant to be definitive. The present sample was relatively small and the analyses may be under-powered for adverse effects. However, HIV treatment success rates remained very high regardless of whether participants quit or did not so any missed events would likely have been small in magnitude. Second, our measures of psychological well-being was restricted to depression and anxiety and, thus, it is possible that other dimensions of psychological well-being that were not assessed worsened among those who quit smoking. Studies in this area typically focus on depression and anxiety symptoms so these results are comparable to the current literature. Finally, the sample was from a randomized clinical trial of varenicline, which required a set of inclusion and exclusion criteria to control for potential confounding factors. As such, the sample is not completely representative of the overall population of smokers with HIV and the results have restricted generalizability.
Nevertheless, the results of this study offer important information for smokers with HIV and their clinicians as they make decisions about using treatments to address tobacco dependence. In particular, the results indicate that less emphasis should be given to worries about quitting smoking interfering with ART adherence or undermining psychological well-being. Consistent with more recent data from the general population, smoking cessation may actually improve psychological well-being as well as reducing physical health risks. Demonstrating this in clinical populations such as PLWHA, where concern about adverse reactions to cessation may be more salient, is critical to increasing the utilization of evidence-based treatments for tobacco dependence and reducing the overall rates of smoking.
Acknowledgments
Funding
This work was supported by National Institutes of Health grants K24 DA045244 and R01 DA033681 and University of Pennsylvania Center for AIDS Research (P30 AI045008) and Penn Mental Health AIDS Research Center (P30 MH097488). Pfizer provided the medication and placebo supply.
Footnotes
Potential Conflicts of Interest
Dr. Schnoll received medication and placebo free from Pfizer and has provided consultation to Pfizer. Dr. Schnoll has provided consultation to GlaxoSmithKline and CuraLeaf. Dr. Gross serves on a DSMB for a Pfizer medication unrelated to smoking or HIV. No other conflicts are declared.
References
- Althoff KN (2016). The shifting paradigm of care for adults living with HIV: Smoking cessation for longer life. The Journal of Infectious Diseases. 214(11), 1618–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Thompson M, Serrano K, Leone F, Metzger D, Frank I,… Schnoll RA (2019a). Placebo-controlled randomized clinical trial testing the efficacy and safety of varenicline for smokers with HIV. Drug and Alcohol Dependence, 200, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Thompson M, Leone F, Metzger D, Gross R, Mounzer K, … Schnoll RA (2019b). Differences in the rate of nicotine metabolism among smokers with and without HIV. AIDS, 33(6),1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin HJ (2009). Management of emergent psychiatric symptoms during smoking cessation. Current Medical Research and Opinion, 25(2), 519–525. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob III P, Ahijevych K, Jarvis MJ, Hall S, & LeHouezec J (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4(2), 149–159. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, & Miller IW (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 12(2), 101–112. [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B & Wu AW (2000). Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care, 12(3), 255–266. [DOI] [PubMed] [Google Scholar]
- Crawford G, Weisbrot J, Bastian J, Flitter A, Jao NC, Carroll A, … Schnoll R (2018). Predictors of varenicline adherence among cancer patients treated for nicotine dependence and its association with smoking cessation. Nicotine & Tobacco Research. Advance online publication. doi: 10.1093/ntr/nty133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drovandi AD, Chen CC, & Glass BD (2016). Adverse effects cause varenicline discontinuation: A meta-analysis. Current Drug Safety, 11, 78–85. [DOI] [PubMed] [Google Scholar]
- Fiore M, Jaén CR, Baker TB, Bailey WC, Benoqitz NL, Curry SJ, … Leitzke C (2008). Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respiratory Care, 53(9), 1217–1222. [PubMed] [Google Scholar]
- Gross R, Yip B, Lo Re V III, Wood E, Alexander CS, Harrigan PR, … Hogg RS (2006). A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. The Journal of Infectious Diseases, 194(8), 1108–1114. [DOI] [PubMed] [Google Scholar]
- Grossberg R, Zhang Y, & Gross R (2004). A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. Journal of Clinical Epidemiology, 57(10), 1107–1110. [DOI] [PubMed] [Google Scholar]
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pederson G, Pederson C, … Obel N (2012). Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clinical Infectious Diseases, 56(5), 727–734. [DOI] [PubMed] [Google Scholar]
- Holmes WC & Shea JA (1998). A new HIV/AIDS-targeted quality of life (HAT-QoL) instrument: development, reliability, and validity. Medical Care, 36(2),138–154. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, & Swan GE (2003). Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research, 5(1), 13–25. [PubMed] [Google Scholar]
- Jarlenski M, Baik SH, & Zhang Y (2016). Trends in use of medications for smoking cessation in medicare, 2007–2012. American Journal of Preventive Medicine, 51(3), 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D, Grasso C, Dant L, Elsesser SA, Crane HM, Cropsey KL, … O’Cleirigh C (2018). Treatment outcomes associated with quitting cigarettes among sexual minority men living with HIV: Antiretroviral adherence, engagement in care, and sustained HIV RNA suppression. AIDS and Behavior, 22(9), 2868–2876. [DOI] [PubMed] [Google Scholar]
- King RM, Vidrine DJ, Danysh HE, Feltcher FE, McCurdy S, Arduino RC, & Gritz ER (2012). Factors associated with nonadherence to antiretroviral therapy in HIV-positive smokers. AIDS Patient Care STDs, 26(8), 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Schnoll RA, Hawk LW, Cinciripini P, George TP, Wileyto EP, … PGRN-PNAT Research Group. (2015). Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. The Lancet Respiratory Medicine, 3(2),131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, & Skarbinski J (2015). Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: Cross-sectional surveys. Annals of Internal Medicine, 162(5), 335–344. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Catley D, Lee HS, & Goggin K (2015). The relationship between ART adherence and smoking status among HIV+ individuals. AIDS and Behavior, 19(4), 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvi S, & Cooperman NA (2009). Review: the need for smoking cessation among HIV-positive smokers. AIDS Education and Prevention, 21(3 Suppl), 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris T, Schiller JS, & Clarke TC (2018). Early Release of Selected Estimates Based on Data From the National Health Interview Survey. https://www.cdc.gov/nchs/nhis/releases.htm. Accessed November 13, 2018.
- Pacek LR, Rass O, & Johnson MW (2017). Positive smoking cessation-related interactions with HIV care providers increase the likelihood of interest in cessation among HIV-positive cigarette smokers. AIDS Care, 29(10), 1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoumenos K & Law MG (2016). Smoking, alcohol and illicit drug use effects on survival in HIV-positive persons. Current Opinion in HIV and AIDS, 11(5), 514–520. [DOI] [PubMed] [Google Scholar]
- Raposeiras-Roubin S, Abu-Assi E, & Iniguez-Romo A (2017). Tobacco, illicit drugs use and risk of cardiovascular disease in patients living with HIV. Current Opinion in HIV and AIDS, 12(6), 523–527. [DOI] [PubMed] [Google Scholar]
- Reddy KP, Parker RA, Losina E, Baggett TP, Paltiel AD, Rigotti NA,… Walensky RP (2016). Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: A US-Based modeling study, The Journal of Infectious Diseases, 214(11), 1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Cano R, Paulus DJ, Zvolensky MJ, López-Durán A, Martínez-Vispo C, & Becoña E (2018). Depressive symptoms in the trajectory of craving during smoking cessation treatment: A latent growth curve model. The American Journal of Drug and Alcohol Abuse, 44(4), 472–479. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, … Hitsman B (2015). Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Internal Medicine, 175(4), 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, & Lerman C (2010). Effectiveness of extended-duration transdermal nicotine therapy: A randomized trial. Annals of Internal Medicine, 152(3), 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab L, Andrew S, & West R (2014). Changes in prevalence of depression and anxiety following smoking cessation: Results from an international cohort study (ATTEMPT). Psychological Medicine, 44(1), 127–141. [DOI] [PubMed] [Google Scholar]
- Shuter J, Salmo LN, Shuter AD, Nivasch EC, Fazzari M & Moadel AB (2012). Provider beliefs and practices relating to tobacco use in patients living with HIV/AIDS: A national survey. AIDS and Behavior, 16(2), 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepankova L, Kralikova E, Zvolska K, Pankova A, Ovesna P, Blaha M, & Brose LS (2017). Depression and smoking cessation: Evidence from a smoking cessation clinic with 1-year follow-up. Annals of Behavioral Medicine, 51(3), 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N & Aveyard P (2014). Change in mental health after smoking cessation: Systematic review and meta-analysis. BMJ, 348:g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandeler G, Johnson LF, & Egger M (2016) Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Current Opinion in HIV and AIDS, 11(5), 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS & Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. [DOI] [PubMed] [Google Scholar]