Abstract
Reproductive mode, ancestry, and climate are hypothesized to determine body size variation in reptiles but their effects have rarely been estimated simultaneously, especially at the intraspecific level. The common lizard (Zootoca vivipara) occupies almost the entire Northern Eurasia and includes viviparous and oviparous lineages, thus representing an excellent model for such studies. Using body length data for >10,000 individuals from 72 geographically distinct populations over the species' range, we analyzed how sex‐specific adult body size and sexual size dimorphism (SSD) is associated with reproductive mode, lineage identity, and several climatic variables. Variation in male size was low and poorly explained by our predictors. In contrast, female size and SSD varied considerably, demonstrating significant effects of reproductive mode and particularly seasonality. Populations of the western oviparous lineage (northern Spain, south‐western France) exhibited a smaller female size and less female‐biased SSD than those of the western viviparous (France to Eastern Europe) and the eastern viviparous (Eastern Europe to Far East) lineages; this pattern persisted even after controlling for climatic effects. The phenotypic response to seasonality was complex: across the lineages, as well as within the eastern viviparous lineage, female size and SSD increase with increasing seasonality, whereas the western viviparous lineage followed the opposing trends. Altogether, viviparous populations seem to follow a saw‐tooth geographic cline, which might reflect the nonmonotonic relationship of body size at maturity in females with the length of activity season. This relationship is predicted to arise in perennial ectotherms as a response to environmental constraints caused by seasonality of growth and reproduction. The SSD allometry followed the converse of Rensch's rule, a rare pattern for amniotes. Our results provide the first evidence of opposing body size—climate relationships in intraspecific units.
Keywords: Bergmann's rule, ecogeographic body size clines, life‐history, lizards, Rensch's rule, Zootoca vivipara
We analyzed the association of adult body size and sexual size dimorphism (SSD) with reproductive mode (viviparity vs. oviparity), lineage identity, and climate in the common lizard (Zootoca vivipara), the species occupying almost entire Northern Eurasia. We revealed a moderate effect of reproductive mode (larger female size and higher SSD in viviparous vs. oviparous populations) and a strong but complex effect of seasonality: female size and SSD decrease with increasing seasonality in the Western viviparous lineage distributed from France to Eastern Europe, while the Eastern viviparous lineage ranging from Eastern Europe to the Far East showed the opposite trends. Our results provide the first evidence of opposing body size—climate relationships in clearly intraspecific units.

1. INTRODUCTION
The patterns and causes of geographic variation in body size are fundamental themes in studies on life‐history evolution (Angilletta, Niewiarowski, Dunham, Leaché, & Porter, 2004; Arendt & Fairbairn, 2012; Roff, 2002). Their importance has further increased in connection with the ongoing climate change, as trends in space may be highly relevant for predictions of changes over time (Gardner, Peters, Kearney, Joseph, & Heinsohn, 2011; Millien et al., 2006; Teplitsky & Millien, 2014). Yet, despite the growing body of publications, the diversity of ecogeographic body size clines remains not fully understood, particularly in ectotherms (see Angilletta, Niewiarowski, et al., 2004; Blanckenhorn & Demont, 2004; Hjernquist et al., 2012; Rypel, 2014; Sears & Angilletta, 2004 for important advances).
Latitudinal and altitudinal clines in body size are the most widely observed ecogeographic patterns (e.g., Ashton & Feldman, 2003; Blanckenhorn & Demont, 2004). Temperature is often assumed to be the principal determinant of ecogeographic body size clines, because temperature covaries consistently with latitude and altitude, and it strongly affects vital processes in the organisms (Angilletta, 2009). Yet, a number of recent studies have found that water availability (precipitation, humidity), and particularly seasonality (within‐year variation in temperature or precipitation), often explain a higher proportion of body size variation than does mean temperature (e.g., Ashton, 2001; Çağlar, Karacaoğlu, Kuyucu, & Sağlam, 2014; Stillwell, Morse, & Fox, 2007). For each of these factors, multiple mechanistic hypotheses have been proposed (see below). However, rigorous testing of such hypotheses is often impeded by collinearity between climatic variables (Millien et al., 2006) which is particularly common within limited geographic areas. Specifically, colder environments are often associated with a greater seasonality (Aragón & Fitze, 2014; Chown & Klok, 2003; Körner, 2000). Furthermore, the pattern of the relationship between a phenotypic trait and a climatic covariate can be nonmonotonic, such as inverted U clines (Hjernquist et al., 2012) or saw‐tooth patterns (Masaki, 1967; Mousseau, 1997). Yet, such more complex patterns are unlikely to be revealed within limited spatial and environmental ranges (Ashton & Feldman, 2003; Gaston, Chown, & Evans, 2008).
Wide‐ranging species present promising models for studying phenotype—climate relationships, because the variation of target traits can be documented for numerous geographically distinct populations exhibiting a wide range of climates and diverse combinations of putative predictors (Roitberg et al., 2013, 2015; cf. Meiri, Yom‐Tov, & Geffen, 2007; Jetz, Ashton, & La Sorte, 2009). The problem is that wide‐ranging species often consist of several phylogeographic lineages. Pooling samples from different lineages may lead to a spurious trait‐climate correlation (Romano & Ficetola, 2010) or obscure true relationships (Ashton, 2001). Therefore, even though body size is both phenotypically plastic and evolutionary malleable (Falconer, 1989; Green & Middleton, 2013; Jetz et al., 2009; Millien et al., 2006), the effects of current environment should be examined jointly with those of ancestry (Ashton, 2004; Diniz‐Filho, 2008; Gaston et al., 2008). Yet, comprehensive range‐wide studies of this kind have rarely been conducted on widespread species, even for fundamentally important traits such as body size (Angilletta, Niewiarowski, et al., 2004; Horváthová et al., 2013; Roitberg et al., 2013). Furthermore, although studies of intraspecific body size variation usually consider both sexes, they seldom explore how body size differences between males and females (i.e., sexual size dimorphism, SSD) vary along geographic or climatic gradients (Laiolo, Illera, & Obeso, 2013; Litzgus & Smith, 2010; Roitberg, 2007; Roitberg et al., 2015; Stillwell et al., 2007). The latter aspect is important because due to sexual differences in reproductive and ecological roles, some factors can affect one sex more than the other. As a result, geographic patterns in body size may differ markedly between males and females (Herczeg, Gonda, & Merilä, 2010; Pearson, Shine, & Williams, 2002; Roitberg et al., 2015; Saino & De Bernardi, 1994; Thorpe & Baez, 1987).
The European common lizard (Zootoca vivipara), one of the most widely distributed terrestrial reptile in the world, is an excellent model for such studies. It occupies almost the entire Northern Eurasia and includes several viviparous and oviparous lineages (clades), three of them inhabiting wide ranges of climates (Figure 1). For this species, there is range‐wide phylogeographic analysis (Surget‐Groba et al., 2006) and extensive data on body size and other life‐history traits for multiple populations (e.g., Bauwens & Verheyen, 1987; Heulin, 1985; Pilorge, 1987). Furthermore, Z. vivipara has become a model species for observational (Chamaillé‐Jammes, Massot, Aragón, & Clobert, 2006; Le Galliard, Marquis, & Massot, 2010; Rutschmann et al., 2016) and experimental (Bestion, Teyssier, Richard, Clobert, & Cote, 2015) studies on how life‐history phenotype may respond to ongoing climate warming. Roitberg et al. (2012, 2013) and Horváthová et al. (2013) studied geographic variation of several life‐history traits in Z. vivipara. However, these studies considered only female size, and they covered the large eastern part of the species range poorly.
Figure 1.

Geographic ranges of different clades of Zootoca vivipara, their phylogenetic relationships (after Surget‐Groba et al., 2006), and our study sites. Details for study samples (1–72) are given in Appendix A7 (Table A1). Two relic lineages (central viviparous I and II), sporadically distributed in the south of Central Europe, are not shown. Note that the distribution border of the eastern oviparous clade (Z. v. carniolica) is actually jagged so that samples 25 and 26 may represent mixtures of different clades (W. Mayer, unpublished data; see also Cornetti et al., 2015; Lindtke et al., 2010) and are excluded from our analyses
The aim of our study was to compile a comprehensive set of body size data for Z. vivipara across Eurasia and estimate the effects of reproductive mode and lineage identity, and the effects of climate on adult body size and sexual size dimorphism in this species. Our specific hypotheses and their predictions are summarized in Table 1 and presented in detail below. Other things being equal, we consider explanations based on plasticity as more parsimonious than hypotheses implying genetic adaptation (Chown & Klok, 2003; Madsen & Shine, 1993; Roitberg et al., 2013).
Table 1.
Summary of specific hypotheses and their predictions tested in our study
| Factor | Proxy | Phenotypic response | Suggested mechanism |
|---|---|---|---|
| Thermal regime during activity season | Mean summer temperature (T2) | Body size increases with T2 (Prediction 1) | Heat acquisition hypothesisa |
| Body size decreases with T2 (Prediction 2) |
(1) Heat conservation hypothesisa (2) Temperature‐size ruleb |
||
| Hydric regime during activity season | Summer precipitation (P2) | Body size decreases with P2 (Prediction 3) |
(1) Dehydration resistance hypothesisa (2) Immediate negative effect of rainfall on lizard activity, food intake, and hence on body growthb |
| Body size increases with P2 (Prediction 4) | Delayed positive effect of rainfall on habitat quality, including food availabilityb | ||
| Length of activity season | Seasonality (here, Mean winter temperature, T1) | Body size increases with T1 (converse pseudo‐Bergmann's cline, Prediction 5) | Adolph and Porter (1993) “null model” (age at maturity is constant)b |
| Body size decreases with T1 (pseudo‐Bergmann's cline, Prediction 6) |
(1) Adolph and Porter (1996) “main model” (modal age at maturity shifts abruptly as season length reaches a threshold)b (2) Starvation resistance hypothesisa |
||
| Sex‐specific effects of cold or seasonal climate | T2 or T1 | Larger female size and converse Rensch's allometry of SSD (Prediction 7a)c | Cold or seasonal climates reduce reproduction frequency, selecting for larger female sizea |
| Smaller male size and standard Rensch's allometry of SSD (Prediction 7b)a, d | Cold or seasonal climates exert energetic constraints on growth and aggressive behavior, thus selecting for smaller male sizea | ||
| Sex‐specific effects of reproductive mode | Oviparous versus viviparous clades | Female size and SSD larger in viviparous forms (Prediction 8)a, e |
Viviparity is associated with: (1) lower reproduction frequency; (2) higher gestation costs; (3) stronger maternal body‐volume constraints on reproductive output |
See text for details and references.
Abbreviation: SSD, sexual size dimorphism.
Hypotheses based on genetic adaptation.
Hypotheses based on plasticity.
Adolph & Porter's “main model” actually predicts a marked decrease of body size with T1 around the threshold values resulting in a saw‐tooth cline whose overall linear trend is decreasing body size with T1.
Female size varies more than male size among populations.
Male size varies more than female size among populations.
1.1. Hypotheses related to temperature
While larger size reduces the surface‐to‐volume ratio, thus better conserving the heat (Bergmann, 1847), smaller size allows getting external heat rapidly (Ashton & Feldman, 2003 and references therein). The latter consideration, which we term heat acquisition hypothesis, is widely accepted for terrestrial ectotherms (Ashton & Feldman, 2003; Oufiero, Gartner, Adolph, & Garland, 2011; Pianka & Vitt, 2003; Pincheira‐Donoso, Hodgson, & Tregenza, 2008). It predicts a converse Bergmann cline, that is, a positive correlation between body size and mean ambient temperature (Prediction 1). Bergmann's (1847) heat conservation hypothesis, predicting a negative correlation between body size and mean ambient temperature (a “standard” Bergmann cline; Prediction 2), is often considered poorly relevant to ectotherms (Cushman, Lawton, & Manly, 1993; Pincheira‐Donoso et al., 2008). Both thermoregulation‐related hypotheses are clearly selectionistic, that is, imply genetic adaptation (e.g., Adams & Church, 2011; Litzgus, DuRant, & Mousseau, 2004).
An alternative but not mutually exclusive hypothesis for Bergmann's clines is the temperature‐size rule, a predominant pattern of developmental plasticity in ectotherms. This rule postulates that individuals growing at lower temperature mature later but at a larger size than conspecifics growing at higher temperature (Angilletta, 2009; Atkinson, 1994).
1.2. Hypotheses related to water availability
Considering that large individuals have a reduced surface‐to‐volume ratio and overall higher absolute water content compared to small individuals, an adaptive dehydration resistance hypothesis predicts a negative correlation of body size with precipitation or humidity (Prediction 3) (see Stillwell et al., 2007 for references). Summer precipitation may also directly affect growth and body size in ectotherms; specifically in lizards and insects, there can be immediate, negative effects on insolation and consequently on animal's activity, food intake and thus body growth and positive, delayed effects on habitat productivity, which enhance foraging opportunity of lizards in later times (reviewed by Çağlar et al., 2014; Le Galliard et al., 2010). The negative effects correspond to our Prediction 3, while the positive ones to Prediction 4.
1.3. Hypotheses related to seasonality
Among multiple hypotheses relating body size variation to the length of annual activity (or inactivity) the models of Adolph and Porter (1993, 1996) seem particularly relevant for our study since they were developed specifically for lizards as perennial terrestrial ectotherms with advanced behavioral thermoregulation. The basic point of their reasoning is that annual growth increment in such organisms is mainly determined by the length of activity season rather than environmental temperature. Their "null physiological model", that is, the basic model assuming the absence of other factors, predicts smaller body size in more seasonal climates (Prediction 5; Adolph & Porter, 1993) which reduce energy acquisition opportunities. This pattern can be termed “converse pseudo‐Bergmann's cline” to distinguish from the true Bergmann's and true converse Bergmann's clines which relate to environmental temperature.
The “main” model by Adolph and Porter (1996) additionally considers a discontinuous variation in the age at maturity (the age at the first reproduction), which is inherent to perennial ectotherms in seasonal climates. It predicts that this variation may reverse the body size cline expected by the null model. As the length of activity season decreases below some threshold, the modal group of subadult individuals cannot attain an appropriate body size within the reproductive season of the same year of life as their conspecifics in an environment allowing longer activity season. Under such constraints, the subadults invest available energy into further growth and start reproduction in the following season. This disproportional prolongation of juvenile growth may overcompensate the shortening of the annual activity period and enhance the typical size at maturity, at least within some range of climates. The predicted pattern is increasing adult body size in more seasonal climates (“pseudo‐Bergmann's” cline; Prediction 6). The main model predicts a saw‐tooth cline (Adolph & Porter, 1996: Figure 4) whose overall linear trend is likely a pseudo‐Bergmann cline as well. As reproduction is expected to more strongly inhibit body growth in females than in males, earlier maturation might be responsible for smaller female relative to male size in warmer or less seasonal climates (see Roitberg & Smirina, 2006 for indirect evidence in another lacertid lizard, Lacerta agilis). Thus, specifically the effect predicted by the main model may be female‐biased. Note that the underlying mechanism of Adolph & Porter's models is a direct response to environmental constraints which is not necessarily accompanied by genetic divergence.
Figure 4.

The saw‐tooth relationship between population's typical adult female size and seasonality in the lizard Zootoca vivipara, as hypothesized from Adolph and Porter's (1993, 1996) models. Thin line segments correspond to constant ages at the first reproduction, where the body size—seasonality relationship follows Adolph and Porter's (1993) null model. Thick segments indicate thresholds at which the age at the first reproduction changes abruptly resulting in a reversed body size—seasonality relationship (Adolph & Porter, 1996). See text for explanations
Prediction 6 is also made by the adaptive fasting endurance, or starvation resistance hypothesis (e.g., Aragón & Fitze, 2014; Ficetola et al., 2010). Its version that applies to temperate zone reptiles explains pseudo‐Bergmann clines via ability of larger‐sized animals to acquire and carry larger fat reserves relative to metabolic needs than smaller‐sized animals, this advantage being more important in climates with longer winters (Ashton, 2001; Litzgus et al., 2004).
1.4. Hypotheses related to sex‐specific selection or plasticity
Two distinct hypotheses related to sex‐specific selection predict more female‐biased SSD in colder and more seasonal climates. The extended fecundity‐advantage hypothesis (reviewed by Cox, Skelly, & John‐Alder, 2003; see also Angilletta, Steury, & Sears, 2004; Litzgus & Smith, 2010; Roitberg et al., 2015) suggests that reduced reproduction frequency should select for higher fecundity and thus for larger females (Prediction 7a).
The small male advantage hypothesis (reviewed by Blanckenhorn, 2000, 2005; Zamudio, 1998; Cox et al., 2003) argues that at low population densities the importance of male–male agonistic interactions, which select for larger body size, should decrease, while the disadvantage of lower mobility (which is often associated with large size) should increase. This hypothesis can be extended for a wider range of conditions. For instance in ectotherms, cold or highly seasonal climates, which reduce energy acquisition opportunities (Congdon, 1989), should also select against energetically costly aggressive behavior (and decrease the benefits of large male size) while increasing the small size‐associated advantage of lower resource demands. Thus, we predict smaller male size in cold or highly seasonal climates (Prediction 7b).
Three related hypotheses predict larger female size and stronger SSD in viviparous versus oviparous forms (Prediction 8). First, like colder environments, viviparity should reduce reproduction frequency, thus selecting for larger females (the extended fecundity‐advantage hypothesis, reviewed by Cox et al., 2003). Second, viviparity may favor larger females via strengthening the maternal body‐volume constraints on reproductive output (reviewed by Roitberg et al., 2013). Third, viviparity enhances costs of pregnancy involving not only physical burden but also metabolic costs (Bleu, Massot, Haussy, & Meylan, 2012; Foucart, Lourdais, DeNardo, & Heulin, 2014; Guillette, 1982). These costs include a marked fecundity‐independent component (Foucart et al., 2014) which may confer additional, survival advantage to larger females (cf. Madsen & Shine, 1994).
Besides sex‐specific selection, ecogeographic clines in SSD may reflect sex‐differential plasticity (Cox & Calsbeek, 2010; Fairbairn, 2005; Hu, Xie, Zhu, Wang, & Lei, 2010; Madsen & Shine, 1993). Albeit the latter hypothesis predicts no particular pattern of body size—climate relationship, it may contribute to explaining an SSD cline when proximate mechanisms of SSD are known or inferable (see Section 4). An important descriptive aspect of body size variation is SSD allometry. This allometry either follows Rensch's rule (male size varies more than female size among populations, resulting in slopes greater than one when log[male size] is regressed on log[female size]) or the opposite, converse Rensch's, pattern (Blanckenhorn, Stillwell, Young, Fox, & Ashton, 2006; Fairbairn, 1997). Specifically, Prediction 7b implies a Rensch's rule, while Predictions 7a and 8 imply its converse.
2. MATERIAL AND METHODS
2.1. Study species
Zootoca vivipara is a small (adult snout‐vent length 40–80 mm), ground‐dwelling, insectivorous, heliothermic lizard. Compared to most other lizards Z. vivipara shows a high resistance to low temperatures and a low resistance to desiccation (Reichling, 1957). It prefers humid habitats, mostly in the forest vegetation zone (see Figure 1 and Roitberg et al., 2013 for further details and references).
2.2. Body size data
We used the snout‐vent length (SVL), the primary measure of body size in lizards and snakes (Roitberg et al., 2011 and references therein), as a proxy for overall structural size. We summarized original and published SVL data for 19,935 common lizards from 240 localities combined in 72 geographically distinct study samples (populations); these cover a major part of the species range (Figure 1; Appendix A7: Table A1). The original data come from museum samples or from previous studies mostly performed for parasitological monitoring (Kuranova et al., 2011). Additional data were extracted from published histograms (e.g., Pilorge, 1987); in few cases, we also considered summary statistics for sex‐specific adult SVL. When data for the same site were found in several studies, all unique samples for each sex were included to increase sample size. In total, 5,055 males and 6,474 females were considered as adults and constituted our study samples (see below).
2.3. Data analysis
Within localities, samples from different years were pooled to increase sample sizes and to apply a standard approach across all data. Whenever reasonable sample sizes were available we used strictly local samples, both for original and published data. When local sample sizes were too small, however, we pooled them into compound samples for larger geographic areas (Figure 1) and used in our analyses weighted means for the study traits and nonweighted means for climatic variables. See Roitberg et al. (2013) for our criteria for pooling samples.
To test robustness of our analyses of the variation in adult body size and SSD to potential confounding factors (see Section 2.4 below) all main analyses were performed for two sets of data. Data set 1 included the animals assigned to adults by the primary researcher (Appendix A7: Table A1, A). Data set 2 was based on single inclusion criteria across samples: body length equal to or exceeding 45 mm for males and 48 mm for females (Table A1, B). These thresholds are close to typical minimum SVL of mature common lizards reported for most viviparous (Avery, 1975; Cavin, 1993; Orlova, 1975; Pilorge & Xavier, 1981) and some oviparous (Sinervo et al., 2007) populations studied. Furthermore, each main analysis was run for mean values and additionally for the 80th percentiles of the size distributions (see Roitberg, 2007 for details and references on higher percentiles as useful estimators of population's typical adult body size in indeterminate growers; see also Case, 1976). Thus, we used four metrics for each of our target traits, that is, male size, female size, and SSD. Means and percentiles of sex‐specific SVL were ln‐transformed for all analyses except SSD.
Sexual size dimorphism was quantified with the index: SDI = (size of larger sex/size of smaller sex) − 1, conventionally expressed as positive if females are larger and negative if males are larger (Lovich & Gibbons, 1992). This index shows several favorable properties (Lovich & Gibbons, 1992; Smith, 1999) and has become a standard metric for studies on sexual dimorphism (Fairbairn, Blanckenhorn, & Szekely, 2007). SSD allometry was quantified with the slope of major axis regression (model II) of log(male SVL) on log(female SVL) (Fairbairn, 1997). The slopes (β) and their 95% confidence intervals were computed with the lmodel2 package (Legendre, 2013) in R (R Core Team, 2018). They were tested against the null hypothesis of β = 1 (isometry). The pattern with β > 1 is referred to as Rensch's rule, and that with β < 1 as converse Rensch's allometry (Table 1).
The following bioclimatic indices were used as explanatory variables: mean temperature of coldest quarter (hereafter T1, winter temperature; Worldclim code BIO11), mean temperature of warmest quarter (T2, summer temperature; BIO10), and precipitation of warmest quarter (P2, summer precipitation; BIO18). T1 is a strong correlate of seasonality (Appendix A3), thus being a reasonable proxy for the length of activity season (Angilletta, Niewiarowski, et al., 2004); variation in T1 reflects the principal direction of climatic variation across temperate Eurasia (Appendix A2). T2 and P2 were our proxies respectively for thermal conditions and water availability during activity season, since the summer months fall into activity periods in virtually all populations. See Appendices A1, A2, A3 for further details on our climatic variables, their covariation patterns, as well as extraction of climate data.
The fourth explanatory variable was clade identity (western oviparous vs. western viviparous vs. eastern viviparous). See Appendix A4 for details and justification of a rough control for ancestry in this study. The effects of spatial autocorrelations and multicollinearity were considered as described in Appendices A5 and A6, respectively.
To simultaneously analyze categorical (clade identity, hereafter Clade) and continuous (climatic variables) effects, as well as their interactions, on the variation among population means (or percentiles) of a target trait, we used general linear models (GLMs). We determined the best combination of predictors of each target trait using an information‐theoretic approach (Burnham & Anderson, 2002) based on the Akaike's Information Criterion corrected for small sample sizes (AICc). Prior to model fitting, all continuous input variables were standardized to mean = 0 and SD = 1 to improve the interpretability of main effects in the presence of significant interactions (Schielzeth, 2010). We then fitted models encompassing all possible combinations of input variables and their first‐order interactions, including an intercept‐only model, calculating for each combination the AICc score. The interactions were included for explorative purposes. Models with ΔAICc ≤ 2 were considered candidate models and used for further analysis. As the Akaike's criterion may select overly complex models, we considered a complex model as a candidate model only if its AICc was lower than AICc of all simpler models nested in the complex model (Richards, Whittingham, & Stephens, 2011). Model selection was performed in R version 3.4.3 using the “MuMIn” package (Bartoń, 2017). Considering collinearity between Clade and T1 (Appendix A6) we refrained from model averaging, as recommended by Freckleton (2011). Instead, we summarized our candidate models verbally. We also performed some additional analyses exploring the effect of clade identity on "climate‐corrected" body size.
To evaluate the effect of reproductive mode the western oviparous clade (the only oviparous clade in our data set) was used as the reference level of the factor Clade. Furthermore, all GLM analyses were repeated for two viviparous clades only. Comparing the effects of Clade in the two data sets (three clades vs. two viviparous clades) provided additional evaluations of the effect of reproductive mode.
Values of all response and explanatory variables for 72 study samples are provided in Appendix A7 (Tables A1 and A2).
2.4. Methodological caveats
In species with continuing growth after maturity, numerous factors unrelated to geographic variation, such as local and temporal fluctuations in the abiotic (e.g., temperature and humidity) and/or biotic (e.g., food resources) environment, can affect patterns of growth, maturation, and survival of different cohorts and thus body size distribution in a particular study sample (Kratochvíl & Frynta, 2002; Roitberg, 2007; Shine, 1994, 2005; Stamps, 1993; Watkins, 1996). Further biases can come from compiling data of several independent researchers. They may differ in measuring routine, type of material (living vs. freshly euthanized vs. preserved specimens), and in their criteria of separating adults from immature animals, that is, inclusion criteria (these can be based e.g., on body size and color pattern vs. the state of gonads). The biases from the first two factors are expected to be within a few percents (Case, 1976; Roitberg et al., 2011; E. S. Roitberg, unpublished data; Vervust, Van Dongen, & Van Damme, 2009), and this is much lower than the observed variation within and among our study samples. Indeed, the factor Type of material was never significant when included in our best models as additional predictor. We also examined potential bias from temporal trends in adult body size (e.g., Chamaillé‐Jammes et al., 2006; Green & Middleton, 2013) by adding the factor Time (1950–1990 vs. 1991–2000 vs. 2001–2015); this addition did not improve our best models. The effects of inclusion criteria, and those of temporal variation in the proportion of newly matured animals (Watkins, 1996), were accounted for by using four different metrics for adult body size (see Section 2.3).
3. RESULTS
3.1. Candidate models for male size
Geographic variation in male SVL was weak (range of sample means 7 mm, min–max 48–55 mm; Appendix A7: Table A1) and poorly explained with our predictors. Even the AICc best‐fit models (hereafter top models) explain only 2%–6% of the total variance (Table 2), and in most analyses they do not perform considerably better than the intercept‐only model (ΔAICc ≤ 2). Only metric 4 explains 17% (Table 2, model 29) and only in one data set.
Table 2.
AICc‐selected models (ΔAICc ≤ 2) for male size (SVL)
| Metric | Model | df | AICc | ΔAICc | Weight | Formula | R 2 | Adj R2 | p |
|---|---|---|---|---|---|---|---|---|---|
| Three‐clade analyses | |||||||||
| M1 | 1 | 4 | −270.34 | 0.00 | 0.167 | T1 + 
|
.079 | .051 | .067 |
| M1 | 2 | 3 | −270.31 | 0.03 | 0.164 |

|
.048 | .033 | .072 |
| M1 | 3 | 3 | −269.42 | 0.92 | 0.105 | T1 | .035 | .021 | .123 |
| M1 | 4 | 3 | −269.30 | 1.05 | 0.099 | P2 | .033 | .019 | .132 |
| M1 | 5 | 2 | −269.14 | 1.21 | 0.091 | (Null) | |||
| M2 | 6 | 3 | −238.54 | 0.00 | 0.270 |

|
.050 | .036 | .064 |
| M2 | 7 | 5 | −237.32 | 1.22 | 0.147 | P2 + T1+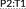
|
.096 | .054 | .087 |
| M2 | 8 | 2 | −237.16 | 1.38 | 0.135 | (Null) | |||
| M2 | 9 | 3 | −237.00 | 1.54 | 0.125 | P2 | .029 | .015 | .162 |
| M3 | 10 | 3 | −257.77 | 0.00 | 0.221 |

|
.055 | .041 | .052 |
| M3 | 11 | 3 | −256.51 | 1.25 | 0.118 | T1 | .038 | .023 | .109 |
| M3 | 12 | 2 | −256.04 | 1.73 | 0.093 | (Null) | |||
| M4 | 13 | 3 | −248.22 | 0.00 | 0.170 |

|
.069 | .055 | .029 |
| M4 | 14 | 4 | −246.28 | 1.94 | 0.065 | T1 + T2 | .073 | .045 | .082 |
| Two‐clade analyses | |||||||||
| M1 | 15 | 2 | −240.01 | 0.00 | 0.154 | (Null) | |||
| M1 | 16 | 3 | −239.60 | 0.42 | 0.125 | Clade | .028 | .012 | .190 |
| M1 | 17 | 3 | −239.58 | 0.43 | 0.124 | T2 | .028 | .012 | .192 |
| M1 | 18 | 3 | −239.55 | 0.47 | 0.122 | P2 | .028 | .012 | .196 |
| M1 | 19 | 3 | −239.39 | 0.62 | 0.113 | T1 | .025 | .009 | .217 |
| M2 | 20 | 2 | −212.71 | 0.00 | 0.136 | (Null) | |||
| M2 | 21 | 3 | −212.51 | 0.20 | 0.123 | T2 | .032 | .016 | .165 |
| M2 | 22 | 3 | −212.17 | 0.54 | 0.104 | T1 | .027 | .010 | .205 |
| M2 | 23 | 3 | −211.96 | 0.75 | 0.094 | Clade | .023 | .007 | .236 |
| M2 | 24 | 3 | −211.94 | 0.77 | 0.093 | P2 | .023 | .007 | .239 |
| M3 | 25 | 3 | −228.77 | 0.00 | 0.217 |

|
.055 | .040 | .065 |
| M3 | 26 | 3 | −227.76 | 1.01 | 0.131 | Clade | .040 | .024 | .120 |
| M3 | 27 | 2 | −227.45 | 1.33 | 0.112 | (Null) | |||
| M3 | 28 | 3 | −227.12 | 1.65 | 0.095 | T1 | .030 | .014 | .178 |
| M4 | 29 | 7 | −223.17 | 0.00 | 0.735 | Clade + P2 + T1 + Clade:P2 + P2:T1 | .236 | .167 | .009 |
Response variables (natural log‐transformed): M1, mean for males with SVL ≥ 45 mm; M2, mean for males defined as “adults” by primary researchers; M3, 80th percentile for males with SVL ≥ 45 mm; M4, 80th percentile for males defined as “adults” by primary researchers. Predictors: Clade, clade identity; T1, mean temperature of coldest quarter (winter temperature); T2, mean temperature of warmest quarter (summer temperature); P2, precipitation of warmest quarter (summer precipitation). Significance of individual predictors: underlined with dots, p < .1; underlined, p < .05; bold, p < .01; underlined bold, p < .001. See Section 2 for details.
3.2. Candidate models for female size
Compared to males, body size variation in females is clearly larger (range of sample means is circa 16 mm, min–max 51–67 mm, Table A1), with much greater part of this variation being explained by our predictors (26%–49%, Table 3). In the three‐clade analyses, all five candidate models include Clade and P2 (summer precipitation); three models include T1 (winter temperature), two of them also including the T1 × P2 interaction (Table 3). In the two‐clade analyses, the top models explain a smaller proportion of the total variance than in the three‐clade analyses (26%–36% vs. 40%–49%, Table 3); they are less consistent among different metrics, and the total number of candidate models is larger (13 vs. 5). Only around half of the candidate models include Clade and P2 (Table 3). T1, which is present in 11 of 13 candidate models in the two‐clade analyses, “replaces” Clade (its collinear counterpart, see Section 2.3) as the most consistent predictor. Model coefficients of T1 were always negative, that is, female size overall increases with decreasing T1. As in the three‐clade analyses, a considerable number of candidate models (5 of 13, three of them being the top models, Table 3) include the P2 × T1 interaction. Note that the latter two predictors are also present in the only significant model for male size (Table 3). Model coefficients of P2 were consistently negative (see also Figure 2c), thus supporting our Prediction 3 (Table 1). The P2 × T1 interaction indicates that the major relationship, female size—T1 (Figure 2a), is modulated by covariate P2: in the eastern viviparous clade, this relationship is stronger at higher than at lower values of P2 (Appendix A8: Figure A2).
Table 3.
AICc‐selected models (ΔAICc ≤ 2) for female size (SVL)
| Metric | Model | df | AICc | ΔAICc | Weight | Formula | R 2 | Adj R2 | p |
|---|---|---|---|---|---|---|---|---|---|
| Three‐clade analyses | |||||||||
| F1 | 1 | 7 | −257.11 | 0.00 | 0.496 |
Clade +  + T1 + P2:T1 + T1 + P2:T1
|
.530 | .492 | 2.7 × 10–9 |
| F1 | 2 | 7 | −256.21 | 0.89 | 0.318 | Clade + T1 + Clade:T1 | .524 | .486 | 4.0 × 10–9 |
| F2 | 3 | 7 | −223.24 | 0.00 | 0.733 |
Clade +  + T1 + P2:T1 + T1 + P2:T1
|
.452 | .409 | 2.7 × 10–7 |
| F3 | 4 | 5 | −234.45 | 0.00 | 0.403 | Clade + P2 | .425 | .398 | 6.7 × 10–8 |
| F4 | 5 | 5 | −226.55 | 0.00 | 0.416 | Clade + P2 | .430 | .404 | 5.1 × 10–8 |
| Two‐clade analyses | |||||||||
| F1 | 6 | 5 | −227.90 | 0.00 | 0.273 | Clade + T1 + Clade:T1 | .387 | .355 | 2.7 × 10–6 |
| F1 | 7 | 5 | −227.52 | 0.38 | 0.227 | P2 + T1 + P2:T1 | .383 | .351 | 3.2 × 10–6 |
| F2 | 8 | 5 | −202.27 | 0.00 | 0.507 | P2 + T1 + P2:T1 | .357 | .324 | 1.0 × 10–5 |
| F2 | 9 | 5 | −200.27 | 2.00 | 0.187 | Clade + T1 + Clade:T1 | .336 | .302 | 2.6 × 10–5 |
| F3 | 10 | 5 | −208.26 | 0.00 | 0.104 |
P2 + T1 + 
|
.293 | .257 | 1.5 × 10–4 |
| F3 | 11 | 4 | −208.08 | 0.18 | 0.095 | Clade + P2 | .263 | .239 | 1.2 × 10–4 |
| F3 | 12 | 5 | −207.51 | 0.75 | 0.072 |
T1 +  + T1:T2 + T1:T2
|
.285 | .248 | 2.1 × 10–4 |
| F3 | 13 | 5 | −207.02 | 1.24 | 0.056 | Clade + T1 + Clade:T1 | .279 | .242 | 2.6 × 10–4 |
| F3 | 14 | 4 | −206.63 | 1.63 | 0.046 | P2 + T1 | .246 | .220 | 2.4 × 10–4 |
| F4 | 15 | 7 | −204.27 | 0.00 | 0.265 | P2 + T1 + T2 +  + T1:T2 + T1:T2
|
.391 | .337 | 2.9 × 10–5 |
| F4 | 16 | 5 | −204.00 | 0.27 | 0.231 | T1 + T2 + T1:T2 | .337 | .303 | 2.5 × 10–5 |
| F4 | 17 | 6 | −202.38 | 1.89 | 0.103 | Clade + P2 + T1 + 
|
.346 | .300 | 6.1 × 10–5 |
| F4 | 18 | 4 | −202.37 | 1.90 | 0.102 | Clade + P2 | .293 | .269 | 3.7 × 10–5 |
Response variables (natural log‐transformed): F1, mean for females with SVL ≥ 48 mm; F2, mean for females defined as “adults” by primary researchers; F3, 80th percentile for females with SVL ≥ 48 mm; F4, 80th percentile for females defined as “adults” by primary researchers. Other designations as in Table 2.
Figure 2.

(a‐c) Female body size (snout‐vent length, SVL) and (d‐f) sexual size dimorphism (SSD) in Zootoca vivipara plotted against three climatic predictors. Regression lines are shown whenever the slopes differ from zero at p < .1
3.3. Candidate models for SSD and the shared patterns of female size and SSD variation
Sexual size dimorphism was consistently female‐biased: SSD index for means varied from 0.04 to 0.27 (Appendix A7: Table A1); that is, females were on average 4%–27% longer in SVL than males. In both the three‐clade and the two‐clade data sets, the major axis regression slope of log(male SVL) on log(female SVL) was significantly lower than 1 (Table 4). This pattern corresponds to a converse of Rensch's rule; that is, the SSD variation is primarily due to variation in female rather than male size.
Table 4.
Major axis regression slopes of male size on female size (log‐transformed mean SVL) among populations within and across lineages of Zootoca vivipara
| Data set | Slope estimate (95% C.I.) | Pearson correlation coefficient (r) between male and female SVL |
|---|---|---|
| All three clades, n = 69 | 0.571 (0.421–0.743) | .663*** |
| Two viviparous clades n = 62 | 0.661 (0.490–0.863) | .684*** |
| Western viviparous, n = 19 | 0.841 (0.422–1.554) | .649** |
| Eastern viviparous, n = 43 | 0.814 (0.591–1.099) | .720*** |
The presented analyses use metric 1 as an estimator of sex‐specific adult body size (see Section 2); using other metrics results in similar patterns.
p < .01,
p < .001.
The variation in SSD is even better explained by our predictors (up to 58%, Table 5) than that of absolute female size. Model selection shows a high consistency in both the three‐ and the two‐clade data sets. None of the top models for SSD includes P2, an important predictor of the female size models (see above). Another discordance with the female size models is a consistent presence of the T1 × T2 interaction, a predictor infrequently occurring in the models for female size. This interaction indicates that in the eastern viviparous clade, as well as for the whole data set, the negative SSD—T1 relationship is stronger at lower than at higher values of T2 (Appendix A8: Figure A3).
Table 5.
AICc‐selected models (ΔAICc ≤ 2) for sexual size dimorphism [SSD, here (female SVL/ male SVL) ‐ 1]
| Metric | Model | df | AICc | ΔAICc | Weight | Formula | R 2 | Adj R 2 | p |
|---|---|---|---|---|---|---|---|---|---|
| Three‐clade analyses | |||||||||
| D1 | 1 | 9 | −287.09 | 0.00 | 0.718 | Clade + T1+T2 + Clade:T1 + T1:T2 | .618 | .575 | 1.0 × 10–10 |
| D2 | 2 | 9 | −264.07 | 0.00 | 0.719 | Clade + T1+T2 + Clade:T1 + T1:T2 | .599 | .553 | 4.0 × 10–10 |
| D2 | 3 | 7 | −262.19 | 1.88 | 0.281 |
Clade +  +T2 + T1:T2 +T2 + T1:T2
|
.556 | .520 | 5.0 × 10–10 |
| D3 | 4 | 9 | −236.94 | 0.00 | 0.632 |
 + T1+T2 + Clade:T1 + T1:T2 + T1+T2 + Clade:T1 + T1:T2
|
.468 | .407 | 1.3 × 10–6 |
| D3 | 5 | 7 | −235.85 | 1.08 | 0.368 | Clade + T1+Clade:T1 | .417 | .370 | 1.7 × 10–6 |
| D4 | 6 | 9 | −240.87 | 0.00 | 0.679 |
 + T1+T2 + Clade:T1 + T1:T2 + T1+T2 + Clade:T1 + T1:T2
|
.517 | .461 | 8.5 × 10–8 |
| Two‐clade analyses | |||||||||
| D1 | 7 | 7 | −257.76 | 0.00 | 0.312 | Clade + T1+T2 + Clade:T1 + T1:T2 | .515 | .471 | 7.0 × 10–8 |
| D1 | 8 | 7 | −256.06 | 1.70 | 0.134 | P2 + T1+T2 + P2:T1 + T1:T2 | .501 | .457 | 1.5 × 10–7 |
| D2 | 9 | 7 | −235.14 | 0.00 | 0.399 |
Clade + T1+T2 +  + T1:T2 + T1:T2
|
.470 | .422 | 7.8 × 10–7 |
| D2 | 10 | 5 | −235.01 | 0.13 | 0.375 | T1 + T2+T1:T2 | .424 | .394 | 4.6 × 10–7 |
| D3 | 11 | 5 | −215.04 | 0.00 | 0.200 | T1 + T2+T1:T2 | .298 | .262 | 1.2 × 10–4 |
| D4 | 12 | 5 | −217.87 | 0.00 | 0.454 | T1 + T2+T1:T2 | .347 | .313 | 1.6 × 10–5 |
The following patterns are common to the female size and the SSD variation. First, candidate models for both traits frequently include the Clade × T1 interaction (Table 3, Table 5) that reflects opposing female size—T1 and SSD—T1 relationships in the two viviparous clades (Figure 2a,d). Both correlations, a positive in the western viviparous clade and a negative in the eastern viviparous clade, are significant for several metrics of female size and SSD, the difference between the two correlation coefficients being significant for all eight metrics (Appendix A8: Table A3). The negative female size—T1 and SSD—T1 correlations for the whole data set are highly significant too (Table A3). The negative correlation corresponds to a pseudo‐Bergmann's cline (Table 1: Prediction 6), while the positive one corresponds to its converse (Table 1: Prediction 5). Second, the main effect of T1 is much stronger than that of T2: (a) while ten models include T1 but not T2, no models show the opposite pattern (Table 3, Table 5); (b) in no model, T2 exhibits a significant main effect. Thus, no support for our Predictions 1 or 2 (Table 1) was found. Third, as with female size, clade identity is a consistent predictor of SSD in the three‐clade analyses (it occurs in all six candidate models, Table 5) and becomes inconsistent in the two‐clade analyses, where four of the six candidate models include climatic predictors only (Table 5); the top models explain a smaller proportion of the total variance in the two‐clade analyses than in the three‐clade analyses (26%–47% vs. 41%–58%, Table 5). To further explore this issue, we visualized between clade differences in female size and SSD as they appear with and without controlling for climatic covariates (Figure 3). In line with Prediction 8 (Table 1), the western oviparous clade exhibits a smaller female size and less female‐biased SSD than the western and the eastern viviparous clades, whereas differences between the two viviparous clades become insignificant when controlling for climatic variables.
Figure 3.

Variation in female size (F = LN[female size]) and sexual size dimorphism (SSD = female size/male size − 1) among three major clades of Zootoca vivipara based on raw values and on residuals of several models which include climatic predictors only. Presented are all models with ΔAICc ≤ 2 and two simpler models (C, H) which are useful for analytical purposes. Models are specified on the Y axes. The presented analyses use metric 1 as an estimator of sex‐specific adult body size (see Section 2); using other metrics results in similar patterns
4. DISCUSSION
We investigated intraspecific divergence of adult body length in a wide‐ranging lizard across the temperate Eurasia. Using general linear models, we tested the effects of mean summer temperature, summer precipitation, seasonality, and reproductive mode/lineage identity on female size, male size, and SSD. We found a moderate effect of reproductive mode and precipitation and a strong but complex effect of seasonality. The latter differed drastically between the lineages, being also modulated by precipitation and especially by temperature. Female size and SSD varied stronger than male size, and virtually all the effects were strongly female‐biased. Below, we relate the revealed body size patterns to several evolutionary and ecological hypotheses (Table 1).
4.1. Allometry of SSD
Amniotes, including reptiles, tend to exhibit standard Rensch's allometry meaning that male size varies more than female size (Fairbairn, 1997). This trend is mainly expressed among species (reviewed in Fairbairn et al., 2007) but was also reported for variation among conspecific populations (e.g., Saino & De Bernardi, 1994; Garel, Solberg, Sæther, Herfindal, & Høgda, 2006; Aglar & López‐Darias, 2016), even in species with overall female‐biased SSD (Pearson et al., 2002). In contrast, SSD variation in Z. vivipara follows a converse of Rensch's rule. In Z. vivipara, male size varies not only lower but also qualitatively less regular in relation to our predictors, as compared to female size. This pattern is not solely due to a divergence between oviparous and viviparous populations (see below), as it persists in our two‐clade analyses (the western + eastern viviparous clades). Obviously, the revealed SSD allometry is also shaped by a steeper slope of the body size—climate relationship in females versus males, a pattern which is opposite to a prevailing trend found in a meta‐analysis of 98 animal species (Blanckenhorn et al., 2006).
4.2. Temperature and water availability during the active season
A strong sexual difference in the extent of body size variation, and in the percentage of this variation explained by our predictors, reduces the relevance of the hypotheses that apply equally to both sexes. This concerns the adaptive hypotheses related to heat acquisition, heat conservation, dehydration resistance, and fasting endurance (Table 1). In accordance with this reasoning, Predictions 1 and 2, which are associated with heat acquisition and heat conservation mechanisms (Table 1), received no support in our analyses: The main effect of the corresponding predictor, mean summer temperature (T2), was consistently weak. The temperature‐size rule, which also makes Prediction 2 (Table 1), thus received no support as well. These results are in line with the notion that in advanced behavioural thermoregulators, ambient temperature may not be as important as the amount of time available for thermoregulation (Adolph & Porter, 1993; Sears & Angilletta, 2004; Uller & Olsson, 2003).
Prediction 3, that is, a negative correlation of body size with summer precipitation (P2), received moderate support in this study. It is shared by the dehydration resistance hypothesis and a negative effect of precipitation on insolation, and thus directly on body growth of heliothermic organisms, such as lizards or insects (Table 1). Previous studies on lizards, including Z. vivipara, made on a small geographic scale in warmer regions (Díaz, Iraeta, Verdú‐Ricoy, Siliceo, & Salvador, 2012; Dunham, 1978; Lorenzon, Clobert, & Massot, 2001; Taylor, 2003) reported a positive effect of precipitation or humidity on body size (Prediction 4). The opposite pattern revealed at a large geographic scale (Figure 2c) could mean that in cooler climates, which occur on a major part of the study species range, the negative, immediate effect of precipitation on animal's activity (Table 1) exceeds the positive, delayed effect on habitat productivity (Table 1).
4.3. Seasonality
The strongest and most interesting pattern revealed in this study concerns the relationship of female size and SSD with mean winter temperature (T1) which is tightly correlated to seasonality and used as proxy for the length of activity season. The western viviparous clade exhibits a converse pseudo‐Bergmann's cline corresponding to the Adolph and Porter (1993) “null physiological model” (Table 1: Prediction 5). In contrast, a standard pseudo‐Bergmann's cline found in the eastern viviparous clade, as well as across the clades, corresponds to their main model (Table 1: Prediction 6) that considers shifts in the age at maturity (Adolph & Porter, 1996). Phenotypic responses predicted by Adolph and Porter (1993, 1996) models can be female‐biased due to sex‐differential plasticity of growth and maturation (Fairbairn, 2005; Cox & John‐Alder, 2007; Cox & Calsbeek, 2010; see also Table 1).
We hypothesize that geographic variation in age at maturity, specifically in females, is minor in the western viviparous clade, while pronounced in the eastern viviparous clade, at least in our data set. Available data on typical age at maturity in different populations of Z. vivipara appear to be in line with this hypothesis. In virtually all western viviparous populations, which were studied demographically, the modal age at maturity is 2 years (Bauwens & Verheyen, 1987; Heulin, 1985; Pilorge, 1987; Pilorge & Xavier, 1981; Strijbosch & Creemers, 1988; S. Hofmann, unpublished data). Demographic data are scarce for the eastern viviparous clade, but the modal age at maturity is expected to be generally higher and apparently more variable than in the western viviparous clade, because its geographic distribution (Figure 1) results in a strongly higher range of experienced seasonality (Figure 2a). Indeed, in the south of West Siberia the typical age at maturity was found to range from 2 to at least 3 years (Bulakhova, Kuranova, & Savelyev, 2007; Epova, Kuranova, Yartsev, & Absalyamova, 2016), being probably even higher in still more severe climates of the Middle and East Siberia. The above considerations allow us to suggest that the opposite correlations of female size (and SSD) with mean winter temperature found in the two major clades of Z. vivipara may reflect a saw‐tooth cline along a seasonality gradient predicted by the Adolph and Porter (1996) model (Figure 4).
A stronger effect of seasonality (as estimated by winter temperature) on female body size and particularly SSD in cooler versus warmer summer climates (T1 × T2 interaction; Tables 3 and 5; see also Appendix A8: Figure A3) is beyond our predictions. Perhaps at lower ambient temperatures, lizards use more of the potential activity period predicted by the Adolph and Porter (1993) “null” model to compensate for process limitations (sensu Congdon, 1989). In contrast, in warmer summer climates, lizards might often reduce their activity to avoid predation risk (Werner & Anholt, 1993; see also Sears, 2005), as well as the risk of overheating and dehydration, which are generally higher in such environments.
The fasting endurance hypothesis (larger individuals possess larger fat reserves and survive hibernation better than smaller individuals – Table 1), which also makes Prediction 6, cannot explain the opposing cline in the western viviparous clade and can hardly integrate a strongly female‐biased phenotypic response.
The extended “small male advantage hypothesis”, which implies a Rensch's allometry of SSD (Prediction 7b), is strongly rejected in the present study that found the opposite, converse Rensch pattern (Table 4). This converse Rensch's allometry (Prediction 7a), as well as the pseudo‐Bergmann cline in female size and SSD, corresponds well with the extended fecundity‐advantage hypothesis viewing this cline as an adaptive compensation of reduced reproduction frequency (Table 1). However, females of our study species produce a single litter per year in a wide range of climates: repeated clutches occur in considerable frequencies only in lowland oviparous populations, which constitute a tiny portion of our study samples (2 from 72); only exceptional cases of multiple clutches per season are known for viviparous populations (Horváthová et al., 2013). At the same time, no evidence for biennial or intermittent breeding so far exists for Z. vivipara. Furthermore, previous work (Roitberg et al., 2013) found no significant relationship of clutch size or mass with seasonality, the relationship of these reproductive traits with summer temperature being negative (Roitberg et al., 2013 applied climatic variables tightly correlated to those used in the present study; see Section 2). The above points impair a straightforward application of extended fecundity‐advantage hypothesis to the body size patterns presented here (this issue will be addressed in our future work). Also, as most other presented hypotheses, the fecundity‐advantage hypothesis does not explain the converse pseudo‐Bergmann's cline in the western viviparous clade.
Thus, the explanation of the body size—seasonality patterns in Z. vivipara based on the Adolph and Porter (1993, 1996) models is clearly more parsimonious and better compatible with the currently available evidence than the possible alternative explanations discussed above and in Appendix A9. The saw‐tooth cline, to which we relate the opposing body size—seasonality relationships in the western and eastern viviparous clades, is a strikingly overlooked detail of the Adolph and Porter (1996) model. Although the authors considered this nonmonotonic phenotypic response as its key prediction (Adolph & Porter, 1996: p. 272), we are unaware of any empirical or theoretic contributions regarding this issue. However, comparable saw‐tooth clines have been reported in some insects where the body size—seasonality relationship shows a converse pseudo‐Bergmann's pattern (as predicted by Adolph & Porter's null physiological model) when number of generations per season remains constant, but reverses it when new generations per season are added (Masaki, 1967; Mousseau, 1997).
The Adolph and Porter (1993, 1996) models obviously have potential to predict the temporal dynamic of characteristic adult body size due to ongoing climate change. Note that a marked increase in mean SVL of reproducing females found in a model Z. vivipara population in southern France over 1988‐2000 (Chamaillé‐Jammes et al., 2006; Le Galliard et al., 2010) corresponds to a converse pseudo‐Bergmann cline, as predicted for increasing activity season length within the same age at maturity (Adolph & Porter, 1993). A further warming can reverse this trend when a major part of yearling females would reproduce (thereby impeding their further growth) because they reach the threshold size within the “reproduction window” of their second calendar year (Adolph & Porter, 1996).
Another important point can be inferred from the revealed patterns of climatic variation. Ashton and Feldman (2003) based their comprehensive meta‐analysis of ecogeographic body size clines in reptiles on mean annual temperature. In our study, mean annual temperature is rather strongly correlated with seasonality and mean winter temperature and only weakly correlated with mean summer temperature (Appendix A3), which might mean that many of the clines reported by Ashton and Feldman (2003) are actually driven by seasonality rather than environmental temperature.
Regardless which mechanism(s) underlay the disparity of body size clines in the western and eastern viviparous clades of Z. vivipara, our study provides the first evidence of opposing body size—climate relationships in clearly intraspecific units. Ashton (2001) found opposing body size clines along seasonality gradients in two closely related allopatric species of rattle snakes, Crotalus oreganus and Crotalus viridis. Another example involves less closely related, yet congeneric iguanian lizards, Sceloporus undulatus and Sceloporus graciosus (Angilletta, Niewiarowski, et al., 2004; Sears & Angilletta, 2004). Remarkably, in all three systems the lineage exhibiting a converse pseudo‐Bergmann cline (the western viviparous clade of Z. vivipara, C. oreganus, S. graciosus) inhabits a western part of the respective continent (Eurasia or North America) and experiences less seasonal climates, while the form showing a standard pseudo‐Bergmann cline (the eastern viviparous clade of Z. vivipara, C. viridis, S. undulatus) lives in more interior parts of the continent and experiences more seasonal climates. Even though these three divergences in body size—climate relationships apparently differ in details, this parallelism deserves more attention, because it may reflect so far unknown factors shaping body size clines in ectotherms. One pattern of this kind has recently been revealed in North American freshwater fish: body size—temperature relationships, whenever significant, were consistently positive in warm‐water species, while consistently negative in cool‐/cold‐water species (Rypel, 2014).
4.4. Reproductive mode and lineage identity
In line with Prediction 8, populations of the western oviparous clade tend to exhibit a smaller female size and less female‐biased SSD than those of both viviparous lineages. This pattern persists when controlling for climatic variables (Figure 3) indicating that clade‐specific properties, rather than only local environment, contribute to the divergent body size phenotype of this form. Albeit phylogenetic effects cannot be fully disregarded here because in terms of ancestry the western oviparous clade is less close to the western and eastern viviparous clades than the two viviparous clades to one another (Surget‐Groba et al., 2006), a distinct reproductive mode is the most likely explanation of this divergence. In congruence, females of the eastern oviparous clade show smaller mean SVL than their viviparous counterparts collected from virtually the same site, be it the western viviparous clade (Lindtke, Mayer, & Böhme, 2010) or a relic clade the central viviparous II (Recknagel et al., 2018). Yet the differences between oviparous and viviparous populations are not clear‐cut and explain a moderate part of the intraspecific variation in female size and SSD in Z. vivipara (Figure 2). This is a likely reason why previous range‐wide studies on Z. vivipara (Horváthová et al., 2013; Roitberg et al., 2013), which had less representative data sets, found no significant effect of reproductive mode on female body size.
A problematic point of this study is evaluating the main effect of clade identity (Clade) on the body size variation among viviparous populations. Clade is collinear with T1 (Appendix A6), our proxy for seasonality, which is the strongest predictor. This collinearity arises due to a very low overlap between the western and eastern viviparous clades along the seasonality gradient (Figure 2a,d) resulting from the west–east separation of their geographic distributions (Figure 1). Further complications come from a significant Clade × T1 interaction (Tables 3 and 5). Under such conditions, the absence of clade identity in some candidate models (Tables 3 and 5), as well as the fact that differences between the two viviparous lineages in female size and SSD (Figure 3a,f) became insignificant when corrected for climatic effects (Figure 3b–e,g,h), are rather suggestive than conclusive evidence of weak or nonexistent main effect of clade identity within viviparous populations. Note that Horváthová et al. (2013) also reported a small effect of ancestry on female body size in Z. vivipara, even though they used a finer control for phylogenetic signal than our study.
5. CONCLUSION
The present study confirms a major role of seasonality (as compared to mean summer temperature and precipitation) in shaping the geographic body size variation as suggested by previous work in Z. vivipara (Horváthová et al., 2013; Roitberg et al., 2013). We show that the body size response to the seasonality gradient is strongly female‐biased, more complex than previously thought, and is parsimoniously interpretable as a saw‐tooth cline along a gradient of activity season lengths predicted by Adolph & Porter's models (Table 1; Figure 4). Within the western viviparous clade occurring from France to Eastern Europe, female size and SSD decrease as seasonality increases. Such a response is predicted under constant age at maturity (Adolph & Porter, 1993), an overall likely condition for this region, judging from its low seasonality and from available demographic data. Within the eastern viviparous clade (Eastern Europe to Far East), as well as across the clades, female size and SSD increase with increasing seasonality. This response is predicted under varying age at maturity (Adolph & Porter, 1996), and such variation, being confirmed by scarce empirical evidence, seems very likely, given the high and strongly variable levels of seasonality of this huge territory. Studies on life‐history and demography of Z. vivipara populations experiencing contrasting levels of seasonality should test whether the opposing body size clines revealed in the two widespread lineages are driven by the mechanisms underlying Adolph & Porter's models. Further, our study demonstrates that not only males (e.g., Aglar & López‐Darias, 2016) but also females can be a driver of intraspecific divergence in amniotes.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
ER designed the study. All authors provided morphometric and other data from their fieldwork or museum samples; SH provided climatic data. ER conducted analyses and wrote the manuscript, to which all coauthors contributed and gave final approval for publication.
ACKNOWLEDGEMENTS
This paper is dedicated to Werner Mayer (1943–2015) whose studies profoundly contributed to the knowledge of genetic differentiation within our study species and to our deceased coauthor Olga Leontyeva (1952–2019). We are grateful to Werner Mayer for advice on lineage composition of Central European samples, Andrey Reshetnikov for assistance with creating Figure 1, and Julia Kavalerchik for developing numerous scripts in perl aiding at processing of our voluminous data. Vladimir Shitikov kindly helped us with AIC‐IT analyses in R; these analyses have benefited from comments by Holger Schielzeth. We thank Astrid Clasen, Michael Fokt, Regina Shamgunova, Igor Tarasov, Vladimir Yakovlev, and Igor Doronin for sharing their SVL data. We also thank the researchers whose published data were compiled for this article. Further thanks go to Frank Tillack (Humboldt Natural History Museum Berlin), Michael Franzen (Zoological State Collections Munig), Markus Auer (Senckenberg Natural History Collections Dresden), Jakob Hallermann (Zoological Museum Hamburg), Irina Dotsenko & Anastasia Malyuk (National Museum of Natural History Kyiv), and Jiří Moravec (National Museum Prague) for access to the collections. Numerous published sources and exact geographic coordinates of several important sites were courtesy of Ulrich Sinsch, Sergey Lyapkov, and Josefa Bleu. Comments of anonymous reviewers resulted in substantial improvement of the quality of the manuscript. This study is part of a larger project supported by the Deutsche Forschungsgemeinschaft (grant RO 4168/1–3 to ESR).
APPENDIX A1.
Extraction and correction of climate data
Climatic data (monthly mean minimum and maximum temperatures, monthly mean precipitation, and several bioclimatic indices) and altitudes for the 240 study localities were obtained from the WorldClim database, version 1.4, which provides values averaged over the years 1950–2000 (Hijmans, Cameron, Parra, Jones, & Jarvis, 2005; www.worldclim.org). We used a grid cell resolution of 30 arc‐seconds and bilinear interpolation as the data extraction settings.
For a few study sites located in mountain regions the altitude value provided by WorldClim deviated substantially (200–500 m) from the value given in the original report, Google Maps or related databases (apparently due to a deviation in the reported coordinate values or local faults within the data base). An established correction for adiabatic cooling (Sears, 2005; Angilletta, Oufiero, & Leaché, 2006; Stillwell et al., 2007; Roitberg et al., 2013) would adjust the temperatures but not the precipitation. To more effectively reduce the resulting deviation in climatic values we used an original approach briefly described below. We generated an array of coordinate values (subsidiary points) around the problematic site. If the discrepancy in altitude is merely due to a small random deviation in coordinate values, as is apparently true for the vast majority of cases, then some of the subsidiary points should be closer to the “true” site than the site with the reported coordinates. Choice of the most relevant point was based on the least difference in the altitude values, the geographic proximity being also accounted for. For virtually all problematic sites of this study, at least one subsidiary point in close proximity provided a sufficiently close altitude value.
APPENDIX A2.
Analysis of climate data
Seasonality was estimated with the coefficient of variation (CV) among monthly values of temperature or precipitations (Dobson & Wigginton, 1996; Ashton, 2001; Çağlar et al., 2014). Temperature data were transformed to Kelvin to allow calculation of the variation coefficient (Ashton, 2001).
The principal component analysis (PCA) was used to summarize the geographic variation of 36 primary climatic variables (monthly mean minimum and maximum temperatures, and monthly mean precipitations): these were reduced to a smaller set of orthogonal vectors, which include a major portion of the total variation. In our previous studies (Roitberg et al., 2013, 2015) the first two principal components, PC1‐clim and PC2‐clim, were directly used as predictors for body size and SSD in statistical models. In this study, we took advantage of the fact that PC1‐clim is tightly correlated to mean temperature of coldest quarter (T1, Worldclim code BIO11) and PC2‐clim to mean temperature of warmest quarter (T2, BIO10) (see Appendix A3). Using these two temperature indices instead of the principal components provides the following advantages. First, T1 and T2 are not confined to a given dataset and hence fully comparable among studies or among subsets of our samples. Second, their values are intuitive. Third, when using T1 and T2, we may extend our list of input variables with precipitation indices. So we included precipitation of warmest quarter (P2, BIO18) to address Predictions 3 and 4 (Table 1).
APPENDIX A3.
Patterns of co‐variation among climatic variables across the study sites
The first axis of the principal component analysis of the climatic variables (PC1‐clim) explained 55.2% of the total variance among localities (Figure A1). PC1‐clim is strongly and positively correlated with all monthly temperature and precipitation parameters outside the warmest quarter (Figure A1); PC1‐clim is highly correlated with T1 (Spearman rank correlation coefficient, rs = .975, p < .001; N = 72) but not T2 (rs = −.016, p = .893). PC1‐clim is also tightly linked to temperature seasonality (rs = −.959, p < .001) and less strongly to precipitation seasonality (rs = −.819, p < .001). As expected, T1 exhibits similar correlations to these seasonality indices (−0.962 and −0.807, respectively). In contrast, the second principal component (PC2‐clim, 28.3% of the total variance, Figure A1) is strongly correlated with the monthly values of the warmer season (April–September), the loadings being consistently positive for temperatures and negative for precipitation (Figure A1). PC2‐clim is tightly related to T2 (rs = .959, p < .001) but not T1 (rs = .122, p = .307). Both PC2‐clim and T2 show no significant relations to temperature and precipitation seasonality (all rs < .21, all p > .09).
Mean annual temperature, the most widely used climatic index, is tightly linked to PC1‐clim, temperature seasonality, and T1 (rs = .913, −.847, and .947, respectively; all p < .001), being weakly correlated to PC2‐clim and T2 (all rs < .4).
Figure A1.
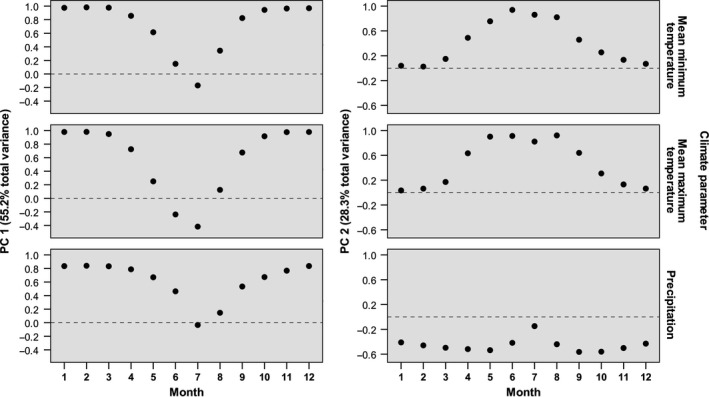
Factor loadings and percents of trace associated with the first two principal components of among‐sites variation in 36 climatic variables
APPENDIX A4.
Considering the effects of evolutionary lineage
The phylogeographic study by Surget‐Groba et al. (2001, 2006) provides reasonably dense covering of border areas between the major clades. Thereby, virtually all our study samples could be readily assigned to particular clades based on their geographic locations (see captions to Figure 1 for two problematic samples). Surget‐Groba et al. (2001, 2006) provided phylogenetic relationships among clades (Figure 1b), but their published data gave only scarce information on the geographical distribution of haplotypes within the clades. Within the viviparous clades, which occupy a major part of the species range, the haplotype variation is apparently small, especially in the eastern viviparous clade (see also Takeuchi, Takeuchi, & Hikida, 2013). This variation is apparently stronger within the western oviparous clade (Milá, Surget‐Groba, Heulin, Gosá, & Fitze, 2013), but our data for this form are insufficient to address this issue. Considering the above circumstances and the fact that there are only three lineages in our study, we included clade identity as a predictor in our analyses (see above) to test for the effects of ancestry, as did Díaz et al. (2012), Roitberg et al. (2013, 2015), Ficetola et al. (2016).
APPENDIX A5.
Considering the effects of spatial autocorrelations
To test whether the results of our GLMs are biased by spatial autocorrelation we computed spline correlograms based on Moran's I statistic; for this we used the ‘ncf’ package (Bjornstad, 2013) in R (R Core Team, 2018). Correlograms were computed for the residuals of the two AICc best‐fit models of each metric of each study trait. No significant autocorrelation was detected (95% confidence intervals always included zero), suggesting that spatial autocorrelation did not bias our models (Dormann et al., 2007).
APPENDIX A6.
Considering the effects of multicollinearity
Multicollinearity between our input variables was tested by computing Generalized Variance Inflation Factor, GVIF (Fox & Monette, 1992) with the car package (Fox & Weisberg, 2011) in R (R Core Team, 2018). In the three‐clade analyses, GVIF amounted 3.98 for clade identity (df = 2), 3.39 for T1, 1.39 for T2, and 1.08 for P2. Despite their higher GVIF values, both T1 and clade identity were retained in our models as these variables have independent biological relevance. Yet, this collinearity was appreciated when processing the results of model selection (see Section 2: Data analysis).
APPENDIX A7.
Data
Table A1.
Summary statistics for snout‐vent length in male and female samples of Zootoca vivipara shaped with two different inclusion criteria (A, B). The values of sexual dimorphism index (Lovich & Gibbons, 1992) for means (SSD_x) and 80th percentiles (SSD_p80) are also given.
| (A) Sample statistics for inclusion criterion 1 (adults as defined by primary researchers) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study sample (see Figure 1 for locations) | Clade | Males, “adults” | Females, “adults” | SSD | ||||||||||||
| ID | Sample details | N | Min | Max | Mean | SD | P80 | N | Min | Max | Mean | SD | P80 | SSD_x | SSD_p80 | |
| 1 | N Spain, W+C Cantabrians | WO | 16 | 43.1 | 54.8 | 49.74 | 3.97 | 54.2 | 21 | 43 | 63 | 52.80 | 5.90 | 59.1 | 0.061 | 0.090 |
| 2 | N Spain, E Cantabrians | WO | 46 | 43.6 | 56.7 | 50.51 | 2.66 | 52.6 | 24 | 49 | 64 | 54.40 | 3.72 | 56.8 | 0.077 | 0.079 |
| 3 | SW France, Pyrenees, Gabas | WO | 54 | 49 | 61 | 54.06 | 2.72 | 56.0 | 180 | 49 | 68 | 58.01 | 4.00 | 62.0 | 0.073 | 0.107 |
| 4 | SW France, Pyrenees, Luvie | WO | 464 | 47 | 59 | 52.93 | 2.14 | 55.0 | 405 | 49 | 63 | 54.91 | 2.91 | 57.0 | 0.037 | 0.036 |
| 5 | SW France, Pyrenees, mixed | WO | 16 | 43 | 53 | 48.08 | 2.80 | 51.0 | 22 | 44 | 62 | 50.28 | 5.43 | 55.4 | 0.046 | 0.087 |
| 6 | SW France, C Pyrenees, N slope | WO | 6 | 43.2 | 55.2 | 48.42 | 4.11 | 52.8 | 26 | 44 | 60 | 52.68 | 4.86 | 57.8 | 0.088 | 0.095 |
| 7 | NE Spain, Pyrenees, Aran Valley | WO | 35 | 43.5 | 54.8 | 48.86 | 2.74 | 51.4 | 88 | 44 | 64 | 53.05 | 3.97 | 56.5 | 0.086 | 0.100 |
| 8 | British Isles | WV | 5 | 52 | 55 | 53.60 | 1.52 | 55.0 | 11 | 49 | 73 | 60.45 | 7.42 | 66.8 | 0.128 | 0.215 |
| 9 | NW France, Paimpont | WV | 144 | 40 | 58 | 51.02 | 3.16 | 54.0 | 208 | 45 | 69 | 58.79 | 4.23 | 63.0 | 0.152 | 0.167 |
| 10 | S France, Besse | WV | 58 | 40 | 59 | 50.19 | 4.12 | 54.0 | 128 | 45 | 70 | 56.31 | 5.30 | 61.0 | 0.122 | 0.130 |
| 11 | S France, Cevennes 1 | WV | 34 | 46.9 | 55.5 | 51.09 | 2.29 | 53.0 | 78 | 49 | 68 | 58.52 | 3.81 | 61.6 | 0.145 | 0.163 |
| 12 | S France, Cevennes 2 | WV | 166 | 42.3 | 59.7 | 51.15 | 3.52 | 54.0 | 238 | 44 | 67 | 54.43 | 4.99 | 59.0 | 0.064 | 0.092 |
| 13 | S France, Cevennes 3 | WV | 50 | 42.6 | 59 | 48.76 | 3.95 | 52.7 | 130 | 44 | 65 | 53.64 | 4.52 | 57.1 | 0.100 | 0.083 |
| 14 | Switzerland, Berner Voralps | WV | 99 | 41 | 58.1 | 48.13 | 3.44 | 51.1 | 87 | 42 | 65 | 54.20 | 4.96 | 58.2 | 0.126 | 0.139 |
| 15 | Belgium, Kalmthout | WV | 182 | 43 | 60 | 51.48 | 3.46 | 54.0 | ||||||||
| 16 | Netherland 1 (Overasselt ) | WV | 205 | 40 | 58 | 48.40 | 3.67 | 51.0 | 195 | 43 | 73 | 55.33 | 6.28 | 61.0 | 0.143 | 0.196 |
| 17 | Netherland 2 (Hammert) | WV | 147 | 41 | 56 | 47.65 | 3.58 | 51.0 | 148 | 41 | 65 | 53.56 | 5.52 | 59.0 | 0.124 | 0.157 |
| 18 | W Germany | WV | 29 | 44.9 | 56.6 | 49.42 | 2.85 | 52.2 | 28 | 41 | 65 | 51.63 | 6.68 | 58.6 | 0.045 | 0.123 |
| 19 | N Germany | WV | 33 | 43.1 | 60 | 50.28 | 3.65 | 52.9 | 26 | 44 | 67 | 55.83 | 6.48 | 62.1 | 0.110 | 0.174 |
| 20 | SW Scandinavia | WV | 17 | 48 | 58 | 54.31 | 2.91 | 57.0 | 17 | 50 | 66 | 58.28 | 5.32 | 63.1 | 0.073 | 0.107 |
| 21 | E Germany | WV | 11 | 46.1 | 53 | 50.71 | 2.26 | 52.9 | 26 | 48 | 65 | 57.04 | 5.10 | 63.2 | 0.125 | 0.195 |
| 22 | E Germany, near Leipzig | WV | 104 | 40 | 60 | 48.96 | 5.15 | 54.0 | 69 | 48 | 71 | 58.51 | 5.66 | 64.0 | 0.195 | 0.185 |
| 23 | C Germany, Thüringia, Altenfeld | WV | 26 | 42 | 56 | 49.37 | 3.03 | 52.0 | 19 | 46 | 60 | 53.17 | 4.20 | 57.0 | 0.077 | 0.096 |
| 24 | N & W Austria + Bayern Alps | WV | 19 | 44 | 61 | 52.27 | 4.27 | 55.2 | 13 | 50 | 65 | 56.60 | 4.59 | 61.2 | 0.083 | 0.109 |
| 25 | S Austria + N Italy | mixed | 13 | 45.6 | 57 | 52.15 | 3.16 | 55.0 | 39 | 47 | 69 | 58.68 | 5.68 | 63.8 | 0.125 | 0.161 |
| 26 | E Austria | mixed | 12 | 42.3 | 57.7 | 51.45 | 3.85 | 53.0 | 12 | 50 | 65 | 58.15 | 4.75 | 63.2 | 0.130 | 0.192 |
| 27 | Czechia | WV | 132 | 40.9 | 59.6 | 48.51 | 3.83 | 51.7 | 118 | 43 | 69 | 53.23 | 5.52 | 57.6 | 0.097 | 0.113 |
| 28 | Slovakia | WV | 45 | 41.5 | 59.8 | 49.23 | 4.13 | 52.3 | 42 | 45 | 64 | 53.91 | 5.23 | 59.4 | 0.095 | 0.134 |
| 29 | S Serbia | WV | 31 | 46.2 | 55 | 50.32 | 2.79 | 53.4 | 45 | 48 | 67 | 55.88 | 4.62 | 60.6 | 0.110 | 0.134 |
| 30 | W Ukraine, Carpathians 1 | EV | 38 | 39.5 | 57 | 46.66 | 4.33 | 51.0 | 36 | 41 | 63 | 53.73 | 5.72 | 59.0 | 0.151 | 0.157 |
| 31 | W Ukraine, Carpathians 2 | EV | 31 | 43 | 58.5 | 50.90 | 3.90 | 54.8 | 27 | 47 | 69 | 58.69 | 5.14 | 63.0 | 0.153 | 0.150 |
| 32 | N Romania, Carpathians 3 | EV | 17 | 43.8 | 54 | 47.31 | 2.84 | 50.3 | 17 | 46 | 60 | 53.45 | 4.08 | 57.3 | 0.130 | 0.139 |
| 33 | lowland W & C Ukraine | EV | 27 | 43 | 58.2 | 52.43 | 3.22 | 55.0 | 15 | 49 | 72 | 59.48 | 6.58 | 66.0 | 0.134 | 0.200 |
| 34 | Finnland | EV | 7 | 44 | 59.3 | 52.80 | 5.71 | 58.2 | 12 | 48 | 69 | 59.09 | 5.72 | 64.0 | 0.119 | 0.100 |
| 35 | near St Petersburg | EV | 46 | 44.3 | 55.7 | 50.52 | 3.28 | 54.0 | 14 | 48 | 66 | 57.30 | 5.58 | 62.2 | 0.134 | 0.152 |
| 36 | Novgorod R, Valday | EV | 63 | 41 | 57 | 48.57 | 3.51 | 52.0 | 122 | 42 | 66 | 55.75 | 5.43 | 60.0 | 0.148 | 0.154 |
| 37 | E Ukraine 1 | EV | 29 | 43 | 57 | 50.36 | 4.00 | 55.0 | 25 | 49 | 71 | 60.38 | 7.06 | 68.8 | 0.199 | 0.251 |
| 38 | E Ukraine 2 | EV | 26 | 43 | 59 | 53.15 | 4.71 | 57.3 | 19 | 47 | 70 | 61.37 | 7.24 | 68.0 | 0.155 | 0.187 |
| 39 | Moscow R | EV | 24 | 44 | 54 | 50.06 | 2.61 | 53.0 | 26 | 51 | 67 | 58.88 | 4.76 | 64.1 | 0.176 | 0.210 |
| 40 | Arkhangelsk R | EV | 24 | 43 | 59.5 | 51.89 | 5.16 | 57.0 | 44 | 51 | 72 | 62.53 | 4.66 | 66.0 | 0.205 | 0.158 |
| 41 | Mordov R | EV | 32 | 41 | 57 | 50.19 | 4.54 | 55.0 | 26 | 43 | 71 | 57.65 | 6.27 | 63.0 | 0.149 | 0.145 |
| 42 | Penza R | EV | 33 | 40 | 56 | 51.52 | 3.90 | 55.0 | 27 | 44 | 70 | 57.19 | 6.98 | 63.4 | 0.110 | 0.153 |
| 43 | Samara R | EV | 28 | 48 | 60 | 53.96 | 3.71 | 58.0 | 24 | 51 | 67 | 58.38 | 5.04 | 65.0 | 0.082 | 0.121 |
| 44 | Perm R, Chepets | EV | 35 | 40 | 57 | 47.37 | 6.01 | 55.0 | 32 | 43 | 68 | 55.47 | 8.37 | 64.4 | 0.171 | 0.171 |
| 45 | Komi R., Pechora‐Ilych nature reserve | EV | 18 | 43.5 | 67.2 | 54.90 | 5.26 | 59.0 | 12 | 54 | 76 | 64.80 | 7.17 | 71.5 | 0.180 | 0.213 |
| 46 | Perm R, Kvazhva | EV | 27 | 42 | 56 | 49.93 | 3.98 | 54.4 | 24 | 49 | 66 | 56.83 | 4.49 | 60.0 | 0.138 | 0.103 |
| 47 | Perm R, Kamenka | EV | 111 | 42 | 60 | 49.67 | 3.33 | 52.6 | 141 | 48 | 72 | 59.00 | 5.41 | 64.0 | 0.188 | 0.217 |
| 48 | W Siberia, North | EV | 10 | 43.7 | 56 | 50.24 | 4.07 | 53.3 | 10 | 48 | 61 | 58.16 | 4.53 | 61.2 | 0.158 | 0.147 |
| 49 | Yugra, SW (Konda) | EV | 15 | 43 | 55 | 47.73 | 3.96 | 52.7 | 20 | 43 | 65 | 53.51 | 6.00 | 59.6 | 0.121 | 0.130 |
| 50 | Yugra, Severnyi | EV | 9 | 44.7 | 52.3 | 48.34 | 2.50 | 50.9 | 14 | 45 | 70 | 60.73 | 7.00 | 66.4 | 0.256 | 0.306 |
| 51 | Yugra, Ob River, W | EV | 36 | 42 | 57 | 49.41 | 3.66 | 53.5 | 37 | 43 | 73 | 56.69 | 7.48 | 64.0 | 0.147 | 0.195 |
| 52 | Yugra, Ob River, mid & E | EV | 41 | 48 | 57 | 52.13 | 2.23 | 54.1 | 38 | 47 | 71 | 59.11 | 5.86 | 64.6 | 0.134 | 0.194 |
| 53 | Yugra, Sibirskiye Uvaly | EV | 40 | 45 | 57 | 50.28 | 3.17 | 53.0 | 27 | 45 | 74 | 57.75 | 6.93 | 63.9 | 0.149 | 0.205 |
| 54 | Krasnoyarsk R | EV | 20 | 42 | 55 | 47.60 | 3.82 | 50.8 | 19 | 49 | 68 | 60.82 | 5.11 | 65.0 | 0.278 | 0.280 |
| 55 | Tomsk R, S taiga | EV | 13 | 48.9 | 60.3 | 52.98 | 2.71 | 54.3 | 85 | 54 | 72 | 62.25 | 4.11 | 65.7 | 0.175 | 0.210 |
| 56 | Tomsk R, Kireyevskoye | EV | 15 | 47.2 | 59 | 53.65 | 3.14 | 56.9 | 30 | 46 | 75 | 61.69 | 5.48 | 65.3 | 0.150 | 0.148 |
| 57 | Tomsk R, Timiryazevo | EV | 173 | 43.8 | 57.8 | 51.81 | 2.91 | 54.2 | 319 | 46 | 71 | 59.18 | 4.20 | 62.7 | 0.142 | 0.156 |
| 58 | Tomsk R, Kuzovlevo | EV | 28 | 46 | 59.7 | 53.03 | 2.90 | 56.0 | 88 | 51 | 70 | 59.60 | 3.88 | 63.0 | 0.124 | 0.125 |
| 59 | Tomsk R, Anikino | EV | 30 | 44.4 | 59 | 49.61 | 3.13 | 51.6 | 32 | 49 | 72 | 57.37 | 5.86 | 62.5 | 0.156 | 0.210 |
| 60 | Kuz. Alatau, N | EV | 7 | 47.6 | 57.7 | 52.47 | 3.60 | 56.1 | 19 | 52 | 68 | 61.09 | 3.74 | 64.2 | 0.164 | 0.145 |
| 61 | Kuz. Alatau, W | EV | 17 | 45 | 61 | 53.63 | 4.05 | 56.1 | 27 | 53 | 76 | 63.36 | 5.38 | 68.0 | 0.181 | 0.212 |
| 62 | Kuz. Alatau, E | EV | 13 | 50.8 | 60 | 55.33 | 2.85 | 58.1 | 23 | 60 | 74 | 67.00 | 4.64 | 72.4 | 0.211 | 0.245 |
| 63 | Kemerovo R, Shoria | EV | 28 | 50.6 | 58.5 | 54.76 | 1.94 | 56.3 | 17 | 56 | 71 | 62.69 | 4.28 | 67.2 | 0.145 | 0.193 |
| 64 | N Altai | EV | 12 | 44.6 | 53.1 | 49.07 | 2.41 | 51.2 | 26 | 47 | 72 | 59.82 | 6.19 | 64.5 | 0.219 | 0.261 |
| 65 | NE Altai, lowland | EV | 45 | 45 | 59 | 51.79 | 2.89 | 54.0 | 41 | 46 | 75 | 60.83 | 6.03 | 66.0 | 0.175 | 0.222 |
| 66 | NE Altai, highland | EV | 21 | 43 | 59 | 52.38 | 4.72 | 57.0 | 64 | 45 | 76 | 60.88 | 7.05 | 67.0 | 0.162 | 0.175 |
| 67 | SW Altai, Markakol | EV | 20 | 43 | 52 | 47.81 | 2.28 | 50.1 | 40 | 46 | 68 | 54.14 | 5.04 | 58.8 | 0.132 | 0.173 |
| 68 | area around Baikal Sea | EV | 20 | 43 | 55.1 | 48.54 | 4.01 | 52.8 | 26 | 49 | 70 | 60.00 | 6.23 | 65.3 | 0.236 | 0.237 |
| 69 | SW Yakutiya | EV | 8 | 42 | 58 | 50.08 | 5.21 | 54.6 | 27 | 49 | 67 | 59.91 | 4.57 | 64.4 | 0.196 | 0.179 |
| 70 | NE China, Sinvu | EV | 42 | 45.8 | 57.3 | 51.86 | 3.13 | 54.7 | 97 | 46 | 72 | 58.69 | 5.44 | 63.6 | 0.132 | 0.162 |
| 71 | Amur R | EV | 26 | 47.6 | 59.6 | 53.26 | 2.94 | 55.4 | 22 | 57 | 68 | 61.67 | 2.63 | 64.0 | 0.158 | 0.155 |
| 72 | S Sakhalin & N Hokkaido | EV | 35 | 43 | 55 | 48.80 | 2.85 | 50.4 | 48 | 47 | 69 | 57.33 | 5.07 | 61.9 | 0.175 | 0.228 |
| (B) Sample statistics for inclusion criterion 2 (males with SVL ≥ 45 mm, females with SVL ≥ 48 mm) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study sample (see Figure 1 for locations) | Clade | Males | Females | SSD | ||||||||||||
| ID | Sample details | N | Min | Max | Mean | SD | P80 | N | Min | Max | Mean | SD | P80 | SSD_x | SSD_p80 | |
| 1 | N Spain, W+C Cantabrians | WO | 13 | 45.7 | 54.8 | 51.11 | 2.96 | 54.3 | 17 | 49 | 63 | 54.76 | 4.59 | 61.1 | 0.071 | 0.124 |
| 2 | N Spain, E Cantabrians | WO | 45 | 45.5 | 56.7 | 50.67 | 2.47 | 53.0 | 24 | 49 | 64 | 54.40 | 3.72 | 56.8 | 0.074 | 0.071 |
| 3 | SW France, Pyrenees, Gabas | WO | 160 | 45 | 61 | 51.73 | 3.89 | 55.0 | 412 | 48 | 68 | 55.84 | 4.55 | 60.0 | 0.079 | 0.091 |
| 4 | SW France, Pyrenees, Luvie | WO | 952 | 45 | 59 | 51.97 | 3.10 | 55.0 | 826 | 48 | 63 | 53.79 | 3.42 | 57.0 | 0.035 | 0.036 |
| 5 | SW France, Pyrenees, mixed | WO | 15 | 45 | 53 | 48.41 | 2.54 | 51.2 | 14 | 48 | 62 | 53.37 | 4.37 | 57.0 | 0.102 | 0.112 |
| 6 | NE Spain, C Pyrenees, N slope | WO | 5 | 45.2 | 55.2 | 49.46 | 3.60 | 54.0 | 22 | 49 | 60 | 54.05 | 3.92 | 58.4 | 0.093 | 0.082 |
| 7 | NE Spain, Pyrenees, Aran Valley | WO | 33 | 45.1 | 54.8 | 49.16 | 2.50 | 51.6 | 82 | 48 | 64 | 53.57 | 3.57 | 56.5 | 0.090 | 0.097 |
| 8 | British Isles | WV | 5 | 52 | 55 | 53.60 | 1.52 | 55.0 | 11 | 49 | 73 | 60.45 | 7.42 | 66.8 | 0.128 | 0.215 |
| 9 | NW France, Paimpont | WV | 335 | 45 | 58 | 51.35 | 3.17 | 54.0 | 594 | 48 | 69 | 59.25 | 4.92 | 63.0 | 0.154 | 0.167 |
| 10 | S France, Besse | WV | 212 | 45 | 59 | 50.70 | 3.58 | 54.0 | 321 | 48 | 70 | 57.69 | 5.50 | 63.0 | 0.138 | 0.167 |
| 11 | S France, Cevennes 1 | WV | 222 | 45 | 55.5 | 50.68 | 3.35 | 54.0 | 260 | 48 | 68 | 57.76 | 6.04 | 63.0 | 0.140 | 0.167 |
| 12 | S France, Cevennes 2 | WV | 571 | 45 | 59.7 | 51.31 | 3.56 | 55.0 | 683 | 48 | 67 | 55.85 | 4.32 | 60.0 | 0.088 | 0.091 |
| 13 | S France, Cevennes 3 | WV | 94 | 45 | 59 | 51.27 | 3.33 | 55.0 | 201 | 48 | 65 | 55.66 | 4.28 | 59.0 | 0.086 | 0.073 |
| 14 | Switzerland, Berner Voralps | WV | 82 | 45 | 58.1 | 49.14 | 2.83 | 52.0 | 77 | 48 | 65 | 55.33 | 4.03 | 58.3 | 0.126 | 0.120 |
| 15 | Belgium, Kalmthout | WV | 157 | 48 | 60 | 52.39 | 2.75 | 54.4 | ||||||||
| 16 | Netherland 1 (Overasselt ) | WV | 172 | 45 | 58 | 49.55 | 2.74 | 51.0 | 169 | 48 | 73 | 56.83 | 5.31 | 61.0 | 0.147 | 0.196 |
| 17 | Netherland 2 (Hammert) | WV | 112 | 45 | 56 | 49.10 | 2.77 | 52.0 | 121 | 48 | 65 | 55.38 | 4.30 | 60.0 | 0.128 | 0.154 |
| 18 | W Germany | WV | 28 | 45.9 | 56.6 | 49.58 | 2.77 | 52.2 | 20 | 48 | 65 | 54.56 | 5.37 | 60.0 | 0.100 | 0.150 |
| 19 | N Germany | WV | 31 | 45 | 60 | 50.69 | 3.36 | 53.1 | 23 | 48 | 67 | 57.10 | 5.71 | 62.2 | 0.126 | 0.172 |
| 20 | SW Scandinavia | WV | 18 | 45.5 | 58 | 53.82 | 3.50 | 57.0 | 17 | 50 | 66 | 58.28 | 5.32 | 63.1 | 0.083 | 0.107 |
| 21 | E Germany | WV | 13 | 45.1 | 53 | 49.92 | 2.83 | 52.8 | 25 | 49 | 65 | 57.42 | 4.81 | 63.6 | 0.150 | 0.205 |
| 22 | E Germany, near Leipzig | WV | 80 | 45 | 60 | 51.09 | 3.81 | 55.0 | 73 | 48 | 71 | 58.10 | 5.81 | 63.2 | 0.137 | 0.149 |
| 23 | C Germany, Thüringia, Altenfeld | WV | 25 | 45.5 | 56 | 49.66 | 2.69 | 52.0 | 18 | 49 | 60 | 53.57 | 3.93 | 57.3 | 0.079 | 0.102 |
| 24 | N & W Austria + Bayern Alps | WV | 17 | 47.8 | 61 | 53.22 | 3.40 | 55.6 | 13 | 50 | 65 | 56.60 | 4.59 | 61.2 | 0.064 | 0.101 |
| 25 | S Austria + N Italy | mixed | 13 | 45.6 | 57 | 52.15 | 3.16 | 55.0 | 36 | 50 | 69 | 59.64 | 4.76 | 63.9 | 0.144 | 0.163 |
| 26 | E Austria | mixed | 11 | 46 | 57.7 | 52.28 | 2.68 | 53.0 | 12 | 50 | 65 | 58.15 | 4.75 | 63.2 | 0.112 | 0.192 |
| 27 | Czechia | WV | 105 | 45.2 | 59.6 | 49.85 | 3.02 | 52.3 | 99 | 48 | 69 | 54.70 | 4.71 | 58.0 | 0.097 | 0.109 |
| 28 | Slovakia | WV | 39 | 45.5 | 59.8 | 50.16 | 3.59 | 53.4 | 37 | 48 | 64 | 54.96 | 4.64 | 59.8 | 0.096 | 0.121 |
| 29 | S Serbia | WV | 31 | 46.2 | 55 | 50.32 | 2.79 | 53.4 | 45 | 48 | 67 | 55.88 | 4.62 | 60.6 | 0.110 | 0.134 |
| 30 | W Ukraine, Carpathians 1 | EV | 25 | 45 | 57 | 49.02 | 3.29 | 52.8 | 31 | 48 | 63 | 55.26 | 4.41 | 59.0 | 0.127 | 0.117 |
| 31 | W Ukraine, Carpathians 2 | EV | 29 | 45 | 58.5 | 51.42 | 3.47 | 55.0 | 26 | 50 | 69 | 59.13 | 4.66 | 63.0 | 0.150 | 0.145 |
| 32 | N Romania, Carpathians 3 | EV | 13 | 45 | 54 | 48.22 | 2.62 | 50.7 | 15 | 49 | 60 | 54.36 | 3.38 | 58.1 | 0.127 | 0.147 |
| 33 | lowland W & C Ukraine | EV | 26 | 48 | 58.2 | 52.79 | 2.66 | 55.0 | 15 | 49 | 72 | 59.48 | 6.58 | 66.0 | 0.127 | 0.200 |
| 34 | Finnland | EV | 6 | 46.4 | 59.3 | 54.27 | 4.59 | 58.5 | 11 | 54 | 69 | 60.12 | 4.70 | 64.6 | 0.108 | 0.104 |
| 35 | near St Petersburg | EV | 45 | 45.2 | 55.7 | 50.70 | 3.10 | 54.0 | 14 | 48 | 66 | 57.30 | 5.58 | 62.2 | 0.130 | 0.152 |
| 36 | Novgorod R, Valday | EV | 54 | 45 | 57 | 49.48 | 2.89 | 52.0 | 115 | 48 | 66 | 56.44 | 4.76 | 60.0 | 0.141 | 0.154 |
| 37 | E Ukraine 1 | EV | 26 | 45.5 | 57 | 51.17 | 3.36 | 55.0 | 25 | 49 | 71 | 60.38 | 7.06 | 68.8 | 0.180 | 0.251 |
| 38 | E Ukraine 2 | EV | 25 | 45.5 | 59 | 53.62 | 4.19 | 57.4 | 19 | 50 | 70 | 61.58 | 6.85 | 68.0 | 0.148 | 0.185 |
| 39 | Moscow R | EV | 22 | 47 | 54 | 50.59 | 1.99 | 53.0 | 26 | 51 | 67 | 58.88 | 4.76 | 64.1 | 0.164 | 0.210 |
| 40 | Arkhangelsk R | EV | 22 | 45.5 | 59.5 | 52.65 | 4.67 | 57.4 | 44 | 51 | 72 | 62.53 | 4.66 | 66.0 | 0.188 | 0.150 |
| 41 | Mordov R | EV | 27 | 45 | 57 | 51.59 | 3.35 | 55.0 | 25 | 50 | 71 | 58.24 | 5.62 | 63.0 | 0.129 | 0.145 |
| 42 | Penza R | EV | 31 | 46 | 56 | 52.16 | 2.99 | 55.0 | 24 | 50 | 70 | 58.63 | 5.96 | 64.0 | 0.124 | 0.164 |
| 43 | Samara R | EV | 28 | 48 | 60 | 53.96 | 3.71 | 58.0 | 24 | 51 | 67 | 58.38 | 5.04 | 65.0 | 0.082 | 0.121 |
| 44 | Perm R, Chepets | EV | 20 | 45 | 57 | 51.50 | 4.58 | 56.0 | 23 | 48 | 68 | 59.83 | 5.22 | 65.0 | 0.162 | 0.161 |
| 45 | Komi R., Pechora‐Ilych nature reserve | EV | 17 | 48.4 | 67.2 | 55.57 | 4.56 | 58.9 | 12 | 54 | 76 | 64.80 | 7.17 | 71.6 | 0.166 | 0.216 |
| 46 | Perm R, Kvazhva | EV | 26 | 45 | 56 | 50.23 | 3.72 | 54.6 | 24 | 49 | 66 | 56.83 | 4.49 | 60.0 | 0.131 | 0.099 |
| 47 | Perm R, Kamenka | EV | 147 | 45 | 60 | 49.91 | 3.05 | 53.0 | 163 | 48 | 72 | 58.99 | 5.39 | 64.0 | 0.182 | 0.208 |
| 48 | W Siberia, North | EV | 8 | 47.4 | 56 | 51.76 | 2.82 | 53.8 | 9 | 54 | 61 | 59.34 | 2.70 | 61.2 | 0.146 | 0.137 |
| 49 | Yugra, SW (Konda) | EV | 12 | 45 | 55 | 48.83 | 3.64 | 53.6 | 16 | 49 | 65 | 55.55 | 4.74 | 60.5 | 0.138 | 0.128 |
| 50 | Yugra, Severnyi | EV | 8 | 46.1 | 52.3 | 48.79 | 2.24 | 51.1 | 13 | 49 | 70 | 61.94 | 5.55 | 66.9 | 0.270 | 0.307 |
| 51 | Yugra, Ob River, W | EV | 32 | 45 | 57 | 50.18 | 3.10 | 53.9 | 32 | 48 | 73 | 58.39 | 6.52 | 64.4 | 0.164 | 0.194 |
| 52 | Yugra, Ob River, mid & E | EV | 42 | 47.9 | 57 | 52.03 | 2.29 | 54.0 | 36 | 50 | 71 | 59.78 | 5.23 | 64.7 | 0.149 | 0.198 |
| 53 | Yugra, Sibirskiye Uvaly | EV | 40 | 45 | 57 | 50.28 | 3.17 | 53.0 | 24 | 51 | 74 | 59.26 | 5.72 | 65.0 | 0.179 | 0.226 |
| 54 | Krasnoyarsk R | EV | 16 | 45 | 55 | 48.74 | 3.36 | 52.5 | 19 | 49 | 68 | 60.82 | 5.11 | 65.0 | 0.248 | 0.238 |
| 55 | Tomsk R, S taiga | EV | 16 | 48.3 | 60.3 | 52.27 | 2.88 | 53.8 | 87 | 49 | 72 | 61.99 | 4.43 | 65.6 | 0.186 | 0.219 |
| 56 | Tomsk R, Kireyevskoye | EV | 19 | 47 | 59 | 52.60 | 3.64 | 56.5 | 33 | 50 | 75 | 61.13 | 5.60 | 65.1 | 0.162 | 0.152 |
| 57 | Tomsk R, Timiryazevo | EV | 196 | 45.2 | 57.8 | 51.39 | 2.98 | 54.0 | 339 | 48 | 71 | 58.77 | 4.45 | 62.3 | 0.144 | 0.154 |
| 58 | Tomsk R, Kuzovlevo | EV | 31 | 46 | 59.7 | 52.43 | 3.32 | 55.5 | 89 | 51 | 70 | 59.50 | 3.97 | 63.0 | 0.135 | 0.134 |
| 59 | Tomsk R, Anikino | EV | 30 | 45.3 | 59 | 49.77 | 2.97 | 51.6 | 37 | 48 | 72 | 56.30 | 6.10 | 61.3 | 0.131 | 0.188 |
| 60 | Kuz. Alatau, N | EV | 7 | 47.6 | 57.7 | 52.47 | 3.60 | 56.1 | 19 | 52 | 68 | 61.09 | 3.74 | 64.2 | 0.164 | 0.145 |
| 61 | Kuz. Alatau, W | EV | 18 | 45 | 61 | 53.52 | 3.96 | 55.8 | 28 | 51 | 76 | 62.93 | 5.74 | 68.0 | 0.176 | 0.219 |
| 62 | Kuz. Alatau, E | EV | 14 | 46.9 | 60 | 54.73 | 3.54 | 58.1 | 24 | 57 | 74 | 66.59 | 4.96 | 72.4 | 0.217 | 0.246 |
| 63 | Kemerovo R, Shoria | EV | 33 | 45.1 | 58.5 | 53.51 | 3.54 | 56.0 | 21 | 54 | 71 | 61.25 | 4.91 | 66.1 | 0.145 | 0.180 |
| 64 | N Altai | EV | 11 | 46 | 53.1 | 49.47 | 2.05 | 51.4 | 26 | 50 | 72 | 59.92 | 5.98 | 64.5 | 0.211 | 0.254 |
| 65 | NE Altai, lowland | EV | 45 | 45 | 59 | 51.79 | 2.89 | 54.0 | 40 | 51 | 75 | 61.20 | 5.62 | 66.0 | 0.182 | 0.222 |
| 66 | NE Altai, highland | EV | 19 | 45 | 59 | 53.32 | 3.87 | 57.0 | 59 | 49 | 76 | 62.17 | 5.67 | 67.0 | 0.166 | 0.175 |
| 67 | SW Altai, Markakol | EV | 19 | 45 | 52 | 48.06 | 2.03 | 50.3 | 36 | 48 | 68 | 54.99 | 4.58 | 59.0 | 0.144 | 0.173 |
| 68 | area around Baikal Sea | EV | 16 | 45.2 | 55.1 | 49.94 | 3.19 | 53.3 | 26 | 49 | 70 | 60.00 | 6.23 | 65.3 | 0.201 | 0.225 |
| 69 | SW Yakutiya | EV | 6 | 48 | 58 | 52.43 | 3.31 | 56.3 | 28 | 49 | 67 | 59.59 | 4.79 | 64.2 | 0.137 | 0.140 |
| 70 | NE China, Sinvu | EV | 42 | 45.8 | 57.3 | 51.86 | 3.13 | 54.8 | 93 | 50 | 72 | 59.18 | 4.99 | 63.5 | 0.141 | 0.159 |
| 71 | Amur R | EV | 26 | 47.6 | 59.6 | 53.26 | 2.94 | 55.4 | 22 | 57 | 68 | 61.67 | 2.63 | 64.0 | 0.158 | 0.155 |
| 72 | S Sakhalin & N Hokkaido | EV | 31 | 45 | 55 | 49.46 | 2.27 | 50.8 | 47 | 49 | 69 | 57.55 | 4.89 | 61.9 | 0.164 | 0.219 |
Data sources: 1, 2: O. A. Arribas; 3, 4: Osenegg, 1995; 5: A. Clasen, E. S. Roitberg; 6, 7: O. A. Arribas; 8: Smith, 2006; Simms, 1969; 9: Heulin, 1985; 10: Pilorge & Xavier, 1981; 11–13: Pilorge, 1987; Khodadoost, Pilorge, & Ortega, 1987; 14: Cavin, 1993; 15: Bauwens & Verheyen, 1987; 16–17: H. Strijbosch et al.; 18–20: E. S. Roitberg, A. Clasen; 21: E. S. Roitberg; 22: S. Hofmann; 23: E. S. Roitberg; 24–26: E. S. Roitberg, A. Clasen; 27–28: M. Fokt; 29: K. Ljubisavljević; 30: E. S. Roitberg; 31: V. F. Orlova, O. I. Zinenko; 32: V. F. Orlova; 33: O. I. Zinenko, V. F. Orlova, E. S. Roitberg; 34: A. Clasen, E. S. Roitberg; 35: E. S. Roitberg; 36: O. A. Leontyeva et al.; 37: O. I. Zinenko, E. S. Roitberg; 38: O. I. Zinenko; 39–40: V. F. Orlova; 41–44: G. V. Eplanova; 45: Anufriev & Bobretsov, 1996; 46: G. V. Eplanova; 47: V. F. Orlova; 48: R. R. Shamgunova, V. F. Orlova; 49–53: R. R. Shamgunova et al.; 54: V. F. Orlova, I. V. Doronin; 55: V. N. Kuranova; 56: V. N. Kuranova; 57: N. A. Bulakhova, V. N. Kuranova; 58: V. N. Kuranova, N. A. Bulakhova; 59: N. A. Bulakhova, V. N. Kuranova; 60: V. N. Kuranova, N. A. Bulakhova; 61: V. N. Kuranova; 62: N. A. Bulakhova, V. N. Kuranova; 63: N. A. Bulakhova; 64: N. A. Bulakhova, V. F. Orlova; 65: V. A. Yakovlev, N. A. Bulakhova; 66: V. A. Yakovlev; 67: V. F. Orlova, E. S. Roitberg; 68: V. F. Orlova; 69: V. F. Orlova, N. A. Bulakhova; 70: Liu, Zhao, Liu, Dong, & Chen, 2008; 71: I. V. Tarasov; 72: V. F. Orlova, I. V. Doronin.
Abbreviations: P80, the 80th percentile; SD, standard deviation.
Geographic abbreviations: N, northern; S, southern; W, western; E, eastern; NW, northwestern; NE, northeastern; SW, southwestern; SE, southeastern; C, central. For Russia, from which a majority of samples comes, the country is not specified but the geographic or administrative region (R) is given. Bayern Alps (part of Sample 24) are in S Germany; Hokkaido (part of Sample 72) is in N Japan.
Table A2.
Geographic and climatic characteristics of study samples
| ID | latitude | longitude | altitude | T1 | T2 | P2 | PC1‐clim | PC2‐clim | S‐t | S‐p | T‐a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42.9335 | −6.0330 | 1622 | 0.5 | 13.4 | 173 | 1.254 | −1.161 | 1.920 | 30.004 | 6.5 |
| 2 | 43.1500 | −3.4330 | 762 | 4.9 | 16.5 | 194 | 1.745 | 0.171 | 1.660 | 23.640 | 10.4 |
| 3 | 42.9000 | −0.4167 | 1165 | 1.0 | 14.4 | 228 | 1.236 | −0.578 | 1.966 | 13.932 | 7.5 |
| 4 | 43.0933 | −0.3792 | 373 | 4.9 | 18.2 | 183 | 1.632 | 1.134 | 1.905 | 15.319 | 11.6 |
| 5 | 42.9967 | −0.3980 | 769 | 3.0 | 16.3 | 206 | 1.436 | 0.273 | 1.934 | 14.199 | 9.6 |
| 6 | 42.9244 | 0.1054 | 2069 | −3.4 | 9.8 | 294 | 0.965 | −2.898 | 1.979 | 12.692 | 2.8 |
| 7 | 42.7610 | 0.9055 | 2103 | −3.2 | 10.3 | 302 | 0.987 | −2.746 | 2.020 | 12.764 | 3.1 |
| 8 | 52.9564 | −3.2919 | 66 | 3.9 | 15.0 | 205 | 1.414 | −0.087 | 1.615 | 21.225 | 9.3 |
| 9 | 48.0833 | −2.3000 | 82 | 5.4 | 16.7 | 172 | 1.500 | 0.877 | 1.630 | 22.292 | 11.1 |
| 10 | 45.5000 | 2.9100 | 1212 | −0.3 | 13.1 | 263 | 0.855 | −0.846 | 1.991 | 19.642 | 6.2 |
| 11 | 44.4500 | 3.7200 | 1405 | −0.8 | 12.7 | 236 | 0.829 | −1.062 | 2.013 | 14.397 | 5.7 |
| 12 | 44.3842 | 3.8778 | 1416 | −0.9 | 12.7 | 237 | 0.848 | −1.108 | 2.026 | 13.779 | 5.7 |
| 13 | 44.3850 | 3.8950 | 1443 | −1.0 | 12.5 | 238 | 0.843 | −1.210 | 2.016 | 13.607 | 5.5 |
| 14 | 46.5470 | 7.7500 | 1443 | −3.1 | 11.9 | 415 | 1.189 | −2.221 | 2.227 | 12.512 | 4.4 |
| 15 | 51.4167 | 4.4167 | 17 | 3.0 | 16.7 | 202 | 1.248 | 0.784 | 1.999 | 13.988 | 9.9 |
| 16 | 51.7780 | 5.8130 | 10 | 2.5 | 16.5 | 217 | 1.132 | 0.764 | 2.038 | 13.777 | 9.6 |
| 17 | 51.5210 | 6.1630 | 21 | 2.6 | 17.0 | 224 | 1.172 | 0.931 | 2.086 | 13.777 | 9.9 |
| 18 | 50.4640 | 6.4963 | 464 | 0.4 | 14.8 | 277 | 1.162 | −0.264 | 2.123 | 14.492 | 7.7 |
| 19 | 54.0828 | 9.8084 | 23 | 0.7 | 16.0 | 226 | 0.897 | 0.494 | 2.234 | 19.880 | 8.3 |
| 20 | 58.2381 | 13.0109 | 144 | −2.4 | 15.1 | 204 | 0.416 | 0.158 | 2.561 | 26.204 | 6.1 |
| 21 | 51.2266 | 13.8346 | 221 | −0.5 | 17.0 | 212 | 0.654 | 1.087 | 2.534 | 26.783 | 8.3 |
| 22 | 51.3670 | 12.2333 | 99 | 0.7 | 17.6 | 174 | 0.638 | 1.479 | 2.436 | 26.927 | 9.1 |
| 23 | 50.5646 | 10.9606 | 632 | −1.9 | 14.6 | 235 | 0.619 | 0.006 | 2.415 | 16.440 | 6.5 |
| 24 | 47.6362 | 11.8636 | 860 | −2.3 | 15.0 | 418 | 0.963 | −0.486 | 2.510 | 34.499 | 6.7 |
| 25 | 46.6485 | 12.4331 | 1585 | −4.3 | 11.6 | 388 | 0.594 | −1.735 | 2.361 | 30.330 | 3.7 |
| 26 | 47.8644 | 16.3059 | 431 | −1.3 | 17.4 | 271 | 0.736 | 0.954 | 2.687 | 33.279 | 8.2 |
| 27 | 49.4868 | 13.9873 | 679 | −2.8 | 14.9 | 293 | 0.660 | −0.105 | 2.570 | 25.051 | 6.3 |
| 28 | 48.9893 | 20.1442 | 778 | −4.0 | 14.6 | 332 | 0.521 | −0.223 | 2.714 | 39.236 | 5.6 |
| 29 | 42.5942 | 21.7525 | 1918 | −5.2 | 10.7 | 223 | 0.438 | −1.734 | 2.365 | 15.354 | 2.9 |
| 30 | 48.6717 | 23.5617 | 642 | −3.5 | 15.7 | 299 | 0.604 | 0.288 | 2.791 | 34.922 | 6.6 |
| 31 | 48.3131 | 23.9084 | 555 | −3.2 | 16.4 | 288 | 0.636 | 0.614 | 2.840 | 36.378 | 7.1 |
| 32 | 47.5217 | 25.5667 | 683 | −3.8 | 16.1 | 286 | 0.428 | 0.670 | 2.899 | 51.425 | 6.7 |
| 33 | 50.0142 | 27.9598 | 215 | −3.9 | 18.3 | 250 | 0.492 | 1.369 | 3.242 | 38.580 | 7.6 |
| 34 | 63.4077 | 26.6587 | 124 | −9.1 | 14.2 | 199 | −0.344 | −0.359 | 3.413 | 31.188 | 2.2 |
| 35 | 60.2225 | 29.6822 | 57 | −6.9 | 16.1 | 217 | 0.074 | 0.257 | 3.359 | 32.001 | 4.5 |
| 36 | 57.9913 | 33.3749 | 209 | −8.8 | 15.5 | 238 | −0.083 | 0.009 | 3.592 | 31.211 | 3.4 |
| 37 | 50.7781 | 34.6514 | 135 | −5.7 | 19.0 | 198 | 0.321 | 1.588 | 3.627 | 24.227 | 6.9 |
| 38 | 49.8183 | 36.5617 | 102 | −5.4 | 19.9 | 169 | 0.359 | 1.984 | 3.709 | 21.152 | 7.6 |
| 39 | 55.3489 | 37.7662 | 153 | −8.1 | 17.4 | 237 | 0.090 | 0.806 | 3.732 | 31.452 | 4.9 |
| 40 | 65.9538 | 42.7954 | 61 | −11.8 | 11.4 | 166 | −0.763 | −1.181 | 3.430 | 30.372 | −0.3 |
| 41 | 54.8350 | 45.3370 | 181 | −10.6 | 18.1 | 193 | −0.239 | 1.077 | 4.249 | 32.212 | 3.9 |
| 42 | 52.7420 | 45.2280 | 207 | −10.3 | 19.1 | 175 | −0.150 | 1.500 | 4.357 | 26.726 | 4.7 |
| 43 | 53.6716 | 50.5661 | 69 | −11.4 | 19.5 | 166 | −0.164 | 1.642 | 4.589 | 24.990 | 4.5 |
| 44 | 60.4258 | 55.6450 | 122 | −14.4 | 15.8 | 222 | −0.556 | −0.147 | 4.429 | 29.933 | .8 |
| 45 | 61.8216 | 56.8420 | 137 | −16.2 | 14.6 | 225 | −0.773 | −0.576 | 4.544 | 30.432 | −0.6 |
| 46 | 58.3830 | 56.4000 | 158 | −13.5 | 16.1 | 223 | −0.477 | 0.173 | 4.364 | 33.962 | 1.7 |
| 47 | 57.9990 | 57.7780 | 262 | −14.4 | 15.6 | 243 | −0.557 | −0.098 | 4.439 | 35.828 | .9 |
| 48 | 64.3000 | 70.4332 | 26 | −21.3 | 13.2 | 196 | −1.523 | −1.094 | 5.197 | 39.763 | −4.3 |
| 49 | 60.4417 | 64.2583 | 53 | −17.1 | 15.9 | 214 | −0.968 | 0.124 | 4.852 | 49.289 | −0.3 |
| 50 | 62.9170 | 72.2330 | 100 | −21.5 | 13.4 | 212 | −1.494 | −1.083 | 5.263 | 41.347 | −4.3 |
| 51 | 61.0278 | 70.1167 | 50 | −19.2 | 15.5 | 220 | −1.145 | −0.223 | 5.158 | 44.841 | −1.7 |
| 52 | 60.9334 | 74.7542 | 34 | −20.1 | 15.2 | 224 | −1.270 | −0.343 | 5.282 | 47.351 | −2.4 |
| 53 | 62.8333 | 81.4167 | 190 | −23.0 | 13.4 | 208 | −1.656 | −1.240 | 5.482 | 39.912 | −5.2 |
| 54 | 60.9859 | 91.5822 | 281 | −24.0 | 14.5 | 202 | −1.508 | −0.861 | 5.779 | 35.239 | −4.5 |
| 55 | 57.6814 | 84.1448 | 96 | −18.1 | 16.3 | 191 | −1.059 | 0.240 | 5.093 | 44.284 | −0.6 |
| 56 | 56.3670 | 84.0830 | 77 | −16.4 | 17.2 | 193 | −0.856 | 0.639 | 4.996 | 41.859 | .7 |
| 57 | 56.4922 | 84.9036 | 73 | −16.1 | 17.3 | 201 | −0.790 | 0.609 | 4.959 | 39.421 | .9 |
| 58 | 56.5667 | 85.0000 | 100 | −16.3 | 17.1 | 204 | −0.805 | 0.511 | 4.953 | 39.080 | .7 |
| 59 | 56.3833 | 85.0164 | 129 | −16.2 | 17.1 | 207 | −0.791 | 0.484 | 4.931 | 38.797 | .7 |
| 60 | 55.5003 | 88.0593 | 453 | −17.0 | 16.0 | 214 | −0.988 | 0.210 | 4.896 | 50.832 | −0.2 |
| 61 | 54.3510 | 87.5900 | 303 | −15.6 | 17.5 | 209 | −0.789 | 0.818 | 4.890 | 53.108 | 1.4 |
| 62 | 54.1798 | 89.2872 | 1073 | −19.0 | 13.5 | 243 | −1.258 | −0.704 | 4.861 | 57.087 | −2.4 |
| 63 | 52.9050 | 87.9145 | 951 | −17.3 | 15.0 | 246 | −1.007 | −0.173 | 4.810 | 58.955 | −0.8 |
| 64 | 51.6019 | 85.6532 | 795 | −14.7 | 16.2 | 220 | −0.766 | 0.400 | 4.601 | 54.824 | 1.1 |
| 65 | 51.8225 | 87.3174 | 455 | −14.6 | 18.4 | 248 | −0.606 | 1.148 | 4.865 | 70.630 | 2.6 |
| 66 | 51.0154 | 88.6153 | 1727 | −18.9 | 12.6 | 167 | −1.519 | −0.516 | 4.719 | 72.760 | −2.7 |
| 67 | 48.7162 | 86.0012 | 1544 | −16.1 | 14.3 | 164 | −1.065 | 0.090 | 4.564 | 53.768 | −0.4 |
| 68 | 53.0241 | 106.9817 | 815 | −21.8 | 14.4 | 262 | −1.517 | −0.301 | 5.420 | 84.292 | −3.0 |
| 69 | 60.3833 | 120.4667 | 202 | −29.7 | 16.0 | 144 | −2.212 | 0.053 | 6.979 | 58.526 | −6.2 |
| 70 | 49.6553 | 127.5695 | 305 | −21.7 | 17.9 | 362 | −1.140 | 0.694 | 5.906 | 104.267 | −0.7 |
| 71 | 49.3375 | 130.3253 | 185 | −24.1 | 18.7 | 368 | −1.079 | 0.832 | 6.387 | 94.214 | −0.9 |
| 72 | 46.6959 | 142.4901 | 55 | −10.0 | 15.3 | 309 | 0.330 | −0.579 | 3.715 | 32.779 | 3.1 |
APPENDIX A8.
Results
Table A3.
Spearman rank correlations (rs) between four different metrics of male size (M1–M4), female size (F1–F4) and SSD (D1–D4) and three climatic variables (T1, T2, and P2)
| Clade | statistic | n | D1‐T1 | D2‐T1 | D3‐T1 | D4‐T1 | F1‐T1 | F2‐T1 | F3‐T1 | F4‐T1 | M1‐T1 | M2‐T1 | M3‐T1 | M4‐T1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Western Oviparous | r s | 7 | −.468 | −.685 | −.450 | −.739 | −.036 | .414 | −.245 | −.234 | .378 | .450 | .136 | .090 |
| p | .289 | .090 | .310 | .058 | .939 | .355 | .596 | .613 | .403 | .310 | .771 | .848 | ||
| Western Viviparous | r s | 19 | .642 | .497 | .663 | .591 | .490 | .332 | .507 | .464 | .084 | .029 | −.070 | .054 |
| p | .003 | .031 | .002 | .008 | .033 | .165 | .027 | .046 | .732 | .906 | .775 | .825 | ||
| Eastern Viviparous | r s | 43 | −.414 | −.254 | −.237 | −.253 | −.377 | −.345 | −.219 | −.222 | −.069 | −.101 | −.012 | .026 |
| p | .006 | .100 | .126 | .102 | .013 | .023 | .158 | .153 | .659 | .517 | .940 | .869 | ||
| All three clades | r s | 69 | −.639 | −.609 | −.444 | −.476 | −.627 | −.573 | −.546 | −.562 | −.189 | −.128 | −.180 | −.198 |
| p | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .119 | .295 | .138 | .102 | ||
| WV versus EV (z) | z | 4.063 | 2.721 | 3.515 | 3.170 | 3.153 | 2.383 | 2.641 | 2.462 | |||||
| WV versus EV (p) | p | .0000 | .003 | .000 | .001 | .002 | .009 | .007 | .004 |
| Clade | Statistic | n | D1‐T2 | D2‐T2 | D3‐T2 | D4‐T2 | F1‐T2 | F2‐T2 | F3‐T2 | F4‐T2 | M1‐T2 | M2‐T2 | M3‐T2 | M4‐T2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Western Oviparous | r s | 7 | −.500 | −.750 | −.464 | −.750 | −.071 | .429 | −.216 | −.214 | .429 | .464 | .198 | .143 |
| p | .253 | .052 | .294 | .052 | .879 | .337 | .641 | .645 | .337 | .294 | .670 | .760 | ||
| Western Viviparous | r s | 19 | .417 | .332 | .470 | .551 | .395 | .296 | .506 | .587 | .043 | −.008 | −.065 | .055 |
| p | .075 | .165 | .042 | .014 | .094 | .218 | .027 | .008 | .861 | .974 | .793 | .824 | ||
| Eastern Viviparous | r s | 43 | −.357 | −.255 | −.103 | −.169 | −.146 | −.015 | −.046 | .017 | .183 | .244 | .144 | .196 |
| p | .019 | .099 | .512 | .279 | .350 | .924 | .772 | .914 | .241 | .115 | .357 | .209 | ||
| All three clades | r s | 69 | .100 | .145 | .193 | .195 | .171 | .236 | .202 | .258 | .219 | .226 | .135 | .217 |
| p | .413 | .236 | .113 | .108 | .160 | .051 | .096 | .032 | .071 | .062 | .270 | .074 |
| Clade | Statistic | n | D1‐P2 | D2‐P2 | D3‐P2 | D4‐P2 | F1‐P2 | F2‐P2 | F3‐P2 | F4‐P2 | M1‐P2 | M2‐P2 | M3‐P2 | M4‐P2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Western Oviparous | r s | 7 | .714 | .714 | .000 | .679 | −.286 | −.107 | −.396 | −.214 | −.536 | −.321 | −.378 | −.321 |
| p | .071 | .071 | 1.000 | .094 | .535 | .819 | .379 | .645 | .215 | .482 | .403 | .482 | ||
| Western Viviparous | r s | 19 | −.504 | −.495 | −.480 | −.514 | −.682 | −.582 | −.642 | −.658 | −.289 | −.267 | −.149 | −.301 |
| p | .028 | .031 | .038 | .024 | .001 | .009 | .003 | .002 | .229 | .270 | .542 | .211 | ||
| Eastern Viviparous | r s | 43 | .065 | .046 | .170 | .180 | −.124 | −.122 | −.164 | −.140 | −.211 | −.160 | −.278 | −.262 |
| p | .679 | .769 | .276 | .248 | .428 | .436 | .294 | .371 | .175 | .304 | .071 | .089 | ||
| All three clades | r s | 69 | −.113 | −.132 | −.068 | −.058 | −.263 | −.268 | −.282 | −.277 | −.281 | −.228 | −.286 | −.310 |
| p | .355 | .280 | .580 | .637 | .029 | .026 | .019 | .021 | .019 | .059 | .017 | .010 |
APPENDIX A9.
Additional hypotheses to explain body‐size—seasonality relationships in common lizards
Body size at maturity is usually tightly related to overall adult size (Shine, 1990; Adolph & Porter, 1996; Stamps, Mangel, & Phillips, 1998). Yet in some perennial ectotherms, geographic patterns or sexual differences in body size at maturity can be strongly modified at later stages via postmaturity growth and survival (Howard, 1981; Rutherford, 2004; Tracy, 1999). In colder and more seasonal climates, perennial ectotherms tend to grow more slowly but live longer than in milder climates. The form of the adult size—seasonality relationship would apparently be determined by the relative strength of these two opposite trends. Obviously, a pseudo‐Bergmann cline more likely arises when the increase in adult survival is “disproportional”, i.e. when the life‐time activity actually increases in seasonal climates instead of being just distributed over more but shorter seasons. Such an increase may occur if the survival increment caused by reduced lizards' potential annual activity (Adolph & Porter, 1993) is added with an extra increment caused by reduced predation and parasite pressure. A corresponding increase in SSD with increasing longevity is expectable if the post‐maturity growth is less decelerated in the larger sex, especially when the larger sex also exhibits a higher adult survival (Watkins, 1996). Both patterns were reported for Zootoca vivipara (growth: Epova et al., 2016; survival: Strijbosch & Creemers, 1988; Uller, Massot, Richard, Lecomte, & Clobert, 2004; Le Galliard et al., 2010). The presented mechanism may strengthen the overall pseudo‐Bergmann trend predicted by the Adolph & Porter (1996) model and explain the increase in female size and SSD with increasing seasonality revealed within the eastern viviparous clade and across the studied clades of Z. vivipara (Figure 2a,d). However, this mechanism cannot explain the opposing cline in the western viviparous clade.
The intriguingly disparate body size—climate relationships in the western and the eastern viviparous clades are difficult to explain outside the Adolph & Porter (1996) model. One alternative scenario would be that the western and eastern viviparous clades differ in their inherited thermal reaction norms (Angilletta, 2009) or potential for response to selection, thus being genetically predisposed to develop opposing body size clines along similar climatic gradients. Currently, such a scenario, implying a substantive role of intrinsic factors, seems unlikely considering that the two clades are closely related (Surget‐Groba et al., 2006) and show no appreciable differences in life‐history (Roitberg et al., 2013) and external morphology (Guillaume et al., 2006).
Figure A2.

The relationship between female body size and winter temperature (T1, our proxy of seasonality) in Zootoca vivipara is modulated by summer precipitation (P2) resulting in a significant T1×P2 interaction (Table 3). Fit line on the right panel indicates a significant relationship (p < .01), the corresponding line on the left panel is given for comparison
Figure A3.
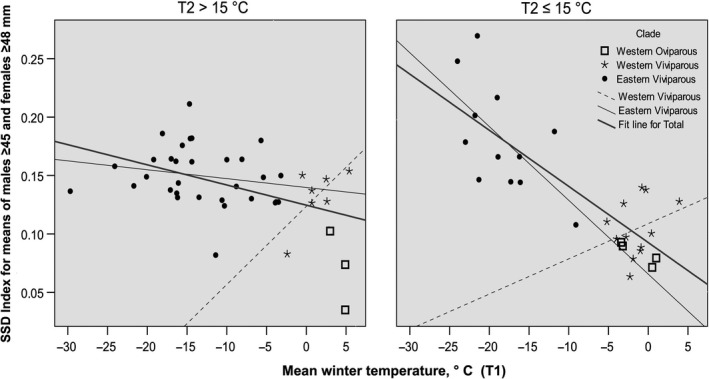
The relationship between sexual size dimorphism (SSD) and winter temperature (T1, our proxy of seasonality) in Zootoca vivipara is modulated by summer temperature (T2) resulting in a significant T1 × T2 interaction (Table 5). Fit lines for the western oviparous clade are not shown because of small sample sizes. Note that the opposite pattern of the SSD—Seasonality relationship in the western and eastern viviparous clades persists in both subsets of populations differing in summer temperature
APPENDIX A10.
References cited in the appendices but not in the main article
- Angilletta, M. J. , Oufiero, C. E. , & Leaché, A. D. (2006). Direct and indirect effects of environmental temperature on the evolution of reproductive strategies: an information‐theoretic approach. The American Naturalist, 168, 123–135. 10.1086/507880 [DOI] [PubMed] [Google Scholar]
- Anufriev, V. M. , & Bobretsov, A. V. (1996). Fauna of the European North‐East of Russia. Vol. 4, Amphibians and Reptiles (133 pp). St. Petersburg, Russia: Nauka Publication. (in Russian). [Google Scholar]
- Bjornstad, O. N. (2013). ncf: spatial nonparametric covariance functions. R package version 1.1‐5. Retrieved from http://CRAN.R-project.org/package=ncf [Google Scholar]
- Dobson, F. S. , & Wigginton, J. D. (1996). Environmental influences on the sexual dimorphism in body size of western bobcats. Oecologia, 108, 610–616. 10.1007/BF00329033 [DOI] [PubMed] [Google Scholar]
- Dormann, C. , McPherson, J. M. , Araújo, M. B. , Bivand, R. , Bolliger, J. , Carl, G. , … Wilson, R. (2007). Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography, 30, 609–628. 10.1111/j.2007.0906-7590.05171.x [DOI] [Google Scholar]
- Ficetola, G. F. , Colleoni, E. , Renaud, J. , Scali, S. , Padoa‐Schioppa, E. , & Thuiller, W. (2016). Morphological variation in salamanders and their potential response to climate change. Global Change Biology, 6, 2013–2024. 10.1111/gcb.13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, J. , & Monette, G. (1992). Generalized collinearity diagnostics. Journal of the American Statistical Association, 7, 178–183. 10.1080/01621459.1992.10475190 [DOI] [Google Scholar]
- Fox, J. , & Weisberg, S. (2011). An R companion to applied regression (2nd ed.). Thousand Oaks, CA: Sage. [Google Scholar]
- Guillaume, C. P. , Heulin, B. , Pavlinov, I. Y. , Semenov, D. V. , Bea, A. , Vogrin, N. , & Surget‐Groba, Y. (2006). Morphological variations in the common lizard, Lacerta (Zootoca) vivipara . Russian Journal of Herpetology, 13, 1–10. 10.30906/1026-2296-2019-13-%25s-1-10 [DOI] [Google Scholar]
- Hijmans, R. J. , Cameron, S. E. , Parra, J. L. , Jones, P. G. , & Jarvis, A. (2005). High resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978. 10.1002/joc.1276 [DOI] [Google Scholar]
- Howard, R. D. (1981). Sexual dimorphism in bullfrogs. Ecology, 62, 303–310. 10.2307/1936704 [DOI] [Google Scholar]
- Khodadoost, M. , Pilorge, T. , & Ortega, A. (1987). Variations de la densité et de la taille corporelle en fonction de la composition du peuplement de proies de trois populations de lézards vivipares du Mont Lozère. Revue d Ecologie, 42, 193–201. [Google Scholar]
- Liu, P. , Zhao, W. G. , Liu, Z. T. , Dong, B. J. , & Chen, H. (2008). Sexual dimorphism and female reproduction in Lacerta vivipara in northeast China. Asiatic Herpetological Research, 11, 98–104. [Google Scholar]
- Milá, B. , Surget‐Groba, Y. , Heulin, B. , Gosá, A. , & Fitze, P. S. (2013). Multilocus phylogeography of the common lizard Zootoca vivipara at the Ibero‐Pyrenean suture zone reveals lowland barriers and high‐elevation introgression. BMC Evolutionary Biology, 13, 192 10.1186/1471-2148-13-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osenegg, K. (1995). Populationsökologische Untersuchungen an der oviparen Form der Waldeidechse, Lacerta (Zootoca) vivipara Jacquin, 1787, im Südwesten Frankreichs. Ph.D. Diss, Bonn Univ. [Google Scholar]
- Rutherford, P. L. (2004). Proximate mechanisms that contribute to female‐biased sexual size dimorphism in an anguid lizard. Canadian Journal of Zoology, 82, 817–822. 10.1139/z04-063 [DOI] [Google Scholar]
- Shine, R. (1990). Proximate determinants of sexual size differences in adult body size. The American Naturalist, 135, 278–283. 10.1086/285043 [DOI] [Google Scholar]
- Simms, C. (1969). Lives of British lizards. Cambridge, UK: Goose & Son. [Google Scholar]
- Smith, M. (1964). The British amphibians and reptiles. London, UK: Collins. [Google Scholar]
- Stamps, J. A. , Mangel, M. , & Phillips, J. A. (1998). A new look at relationships between size at maturity and asymptotic size. The American Naturalist, 152, 470–479. 10.1086/286183 [DOI] [PubMed] [Google Scholar]
- Surget‐Groba, Y. , Heulin, B. , Guillaume, C.‐P. , Thorpe, R. S. , Kupriyanova, L. , Vogrin, N. , … Smith, N. (2001). Intraspecific phylogeography of Lacerta vivipara and the evolution of viviparity. Molecular Phylogenetics and Evolution, 18, 449–459. 10.1006/mpev.2000.0896 [DOI] [PubMed] [Google Scholar]
- Takeuchi, H. , Takeuchi, M. , & Hikida, T. (2013). Extremely low genetic diversity in the Japanese population of Zootoca vivipara (Squamata: Lacertidae) revealed by mitochondrial DNA. Current Herpetology, 32, 66–70. 10.5358/hsj.32.66 [DOI] [Google Scholar]
- Tracy, C. R. (1999). Differences in body size among chuckwalla (Sauromalus obesus) populations. Ecology, 80, 259–271. 10.1890/0012-9658(1999)080[0259:DIBSAC]2.0.CO;2 [DOI] [Google Scholar]
- Uller, T. , Massot, M. , Richard, M. , Lecomte, J. , & Clobert, J. (2004). Long‐lasting fitness consequences of prenatal sex ratio in a viviparous lizard. Evolution, 58, 2511–2516. 10.1111/j.0014-3820.2004.tb00880.x [DOI] [PubMed] [Google Scholar]
Roitberg ES, Orlova VF, Bulakhova NA, et al. Variation in body size and sexual size dimorphism in the most widely ranging lizard: testing the effects of reproductive mode and climate. Ecol Evol. 2020;10:4531–4561. 10.1002/ece3.6077
Olga A. Leontyeva passed away on 27th March 2019.
DATA AVAILABILITY STATEMENT
Original individual‐based data on body size (SVL), as well as the geographic and climatic data, are archived into the public repository Dryad https://doi.org/10.5061/dryad.r7sqv9s7v.
REFERENCES
- Adams, D. C. , & Church, J. O. (2011). The evolution of large‐scale body size clines in Plethodon: evidence of heat‐balance or species‐specific artifact? Ecography, 34, 1067–1075. 10.1111/j.1600-0587.2011.06911.x [DOI] [Google Scholar]
- Adolph, S. C. , & Porter, W. P. (1993). Temperature, activity, and lizards life histories. The American Naturalist, 142, 273–295. 10.1086/285538 [DOI] [PubMed] [Google Scholar]
- Adolph, S. C. , & Porter, W. P. (1996). Growth, seasonality, and lizards life histories. Oikos, 77, 267–278. 10.2307/3546065 [DOI] [Google Scholar]
- Aglar, C. , & López‐Darias, M. (2016). Sex‐specific responses of phenotypic diversity to environmental variation. Ecography, 39, 715–725. 10.1111/ecog.01696 [DOI] [Google Scholar]
- Angilletta, M. J. (2009). Thermal adaptation: a theoretical and empirical synthesis. Oxford:Oxford University Press; 10.1093/acprof:oso/9780198570875.001.1 [DOI] [Google Scholar]
- Angilletta, M. J. , Niewiarowski, P. H. , Dunham, A. E. , Leaché, A. , & Porter, W. P. (2004). Bergmann's clines in ectotherms: illustrating a life‐historical perspective with sceloporine lizards. The American Naturalist, 164, E168–E183. 10.1086/425222 [DOI] [PubMed] [Google Scholar]
- Angilletta, M. J. Jr , Steury, T. D. , & Sears, M. W. (2004). Temperature, growth rate, and body size in ectotherms: fitting pieces of a life‐history puzzle. Integrative & Comparative Biology, 44, 498–509. 10.1093/icb/44.6.498 [DOI] [PubMed] [Google Scholar]
- Aragón, P. , & Fitze, P. S. (2014). Geographical and temporal body size variation in a reptile: roles of sex, ecology, phylogeny and ecology structured in phylogeny. PLoS ONE, 9(8), e104026 10.1371/journal.pone.0104026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt, J. , & Fairbairn, D. J. (2012). Reproductive allometry does not explain the temperature‐size rule in water striders (Aquarius remigis). Evolutionary Ecology, 26, 745–757. 10.1007/s10682-011-9524-4 [DOI] [Google Scholar]
- Ashton, K. G. (2001). Body size variation among mainland populations of the western rattlesnake (Crotalus viridis). Evolution, 55, 2523–2533. [DOI] [PubMed] [Google Scholar]
- Ashton, K. G. (2004). Comparing phylogenetic signal in intraspecific and interspecific body size datasets. Journal of Evolutionary Biology, 17, 1157–1163. 10.1111/j.1420-9101.2004.00764.x [DOI] [PubMed] [Google Scholar]
- Ashton, K. G. , & Feldman, C. R. (2003). Bergmann's rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution, 57, 1151–1163. 10.1111/j.0014-3820.2003.tb00324 [DOI] [PubMed] [Google Scholar]
- Atkinson, D. (1994). Temperature and organism size: a biological law for ectotherms? Advances in Ecological Research, 25, 1–58. 10.1016/S0065-2504(08)60212-3 [DOI] [Google Scholar]
- Avery, R. A. (1975). Clutch‐size and reproductive effort in the lizard Lacerta vivipara Jacquin. Oecologia, 19, 165–170. 10.1007/BF00369099 [DOI] [PubMed] [Google Scholar]
- Bartoń, K. (2017). MuMIn: multi‐model inference. R package version 1.15.1. Retrieved from https://cran.r-project.org/web/packages/MuMIn [Google Scholar]
- Bauwens, D. , & Verheyen, R. F. (1987). Variation of reproductive traits in a population of the lizard Lacerta vivipara . Holarctic Ecology, 10, 120–127. 10.1111/j.1600-0587.1987.tb00748.x [DOI] [Google Scholar]
- Bergmann, C. (1847). Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien, 1, 595–708. [Google Scholar]
- Bestion, E. , Teyssier, A. , Richard, M. , Clobert, J. , & Cote, J. (2015). Live fast, die young: experimental evidence of population extinction risk due to climate change. PLoS Biology, 13, e1002281 10.1371/journal.pbio.1002281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckenhorn, W. U. (2000). The evolution of body size: what keeps organisms small? The Quarterly Review of Biology, 75, 385–407. 10.1086/393620 [DOI] [PubMed] [Google Scholar]
- Blanckenhorn, W. U. (2005). Behavioral causes and consequences of sexual size dimorphism. Ethology, 111, 977–1016. 10.1111/j.1439-0310.2005.01147.x [DOI] [Google Scholar]
- Blanckenhorn, W. U. , & Demont, M. (2004). Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integrative and Comparative Biology, 44, 413–424. 10.1093/icb/44.6.413 [DOI] [PubMed] [Google Scholar]
- Blanckenhorn, W. U. , Stillwell, R. C. , Young, K. A. , Fox, C. W. , & Ashton, K. G. (2006). When Rensch meets Bergmann: does sexual size dimorphism change systematically with latitude? Evolution, 60, 2004–2011. 10.1111/j.0014-3820.2006.tb01838.x [DOI] [PubMed] [Google Scholar]
- Bleu, J. , Massot, M. , Haussy, C. , & Meylan, S. (2012). Experimental litter size reduction reveals costs of gestation and delayed effects on offspring in a viviparous lizard. Proceeding of the Royal Society B, 279, 489–498. 10.1098/rspb.2011.0966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulakhova, N. A. , Kuranova, V. N. , & Savelyev, S. V. (2007). Some demographic characteristics of populations of the sand lizard (Lacerta agilis L., 1758) and the common lizard (Zootoca vivipara (Jacquin, 1787)) in the south‐eastern West Siberia. Vestnik of the Tomsk University, Ser. Biol., № 1: 50–66.
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodel inference. A practical information‐theoretic approach (2nd ed.). New York: Springer Publ; 10.1007/b97636 [DOI] [Google Scholar]
- Çağlar, S. S. , Karacaoğlu, Ç. , Kuyucu, A. C. , & Sağlam, İ. K. (2014). Humidity and seasonality drives body size patterns in males of the bush cricket Isophya rizeensis Sevgili, 2003 (Orthoptera: Tettigoniidae: Phaneropterinae). Insect Science, 21, 213–226. 10.1111/1744-7917.12027 [DOI] [PubMed] [Google Scholar]
- Case, T. J. (1976). Body size differences between populations of the chuckwalla, Sauromalus obesus . Ecology, 57, 313–323. 10.2307/1934819 [DOI] [Google Scholar]
- Cavin, L. (1993). Structure d'une population subalpine de Lézards vivipares (Lacerta vivipara Jacquin, 1787). Revue Suisse De Zoologie, 100, 357–371. 10.5962/bhl.part.79866 [DOI] [Google Scholar]
- Chamaillé‐Jammes, S. , Massot, M. , Aragón, P. , & Clobert, J. (2006). Global warming and positive fitness response in mountain populations of common lizards Lacerta vivipara . Global Change Biology, 12, 392–402. 10.1111/j.1365-2486.2005.01088.x [DOI] [Google Scholar]
- Chown, S. L. , & Klok, C. J. (2003). Altitudinal body size clines: latitudinal effects associated with changing seasonality. Ecography, 26, 445–455. 10.1034/j.1600-0587.2003.03479.x [DOI] [Google Scholar]
- Congdon, J. D. (1989). Proximate and evolutionary constraints on energy relations of reptiles. Physiological Zoology, 62, 356–373. 10.1086/physzool.62.2.30156175 [DOI] [Google Scholar]
- Cornetti, L. , Belluardo, F. , Ghielmi, S. , Giovine, G. , Ficetola, G. F. , Bertorelle, G. , … Hauffe, H. C. (2015). Reproductive isolation between oviparous and viviparous lineages of the Eurasian common lizard Zootoca vivipara in a contact zone. Biological Journal of the Linnean Society, 114, 566–573. 10.1111/bij.12478 [DOI] [Google Scholar]
- Cox, R. M. , & Calsbeek, R. (2010). Sex‐specific selection and intraspecific variation in sexual size dimorphism. Evolution, 64, 798–809. 10.1111/j.1558-5646.2009.00851.x [DOI] [PubMed] [Google Scholar]
- Cox, R. M. , & John‐Alder, H. B. (2007). Growing apart together: the development of contrasting sexual size dimorphisms in sympatric Sceloporus lizards. Herpetologica, 63, 245–257. 10.1655/0018-0831(2007)63[245:GATTDO]2.0.CO;2 [DOI] [Google Scholar]
- Cox, R. M. , Skelly, S. L. , & John-Alder, H. B. (2003). A comparative test of adaptive hypotheses for sexual size dimorphism in lizards. Evolution, 57, 1653–1669. [DOI] [PubMed] [Google Scholar]
- Cushman, J. H. , Lawton, J. H. , & Manly, B. F. J. (1993). Latitudinal patterns in European ant assemblages: variation in species richness and body size. Oecologia, 95, 30–37. 10.1007/BF00649503 [DOI] [PubMed] [Google Scholar]
- Díaz, J. A. , Iraeta, P. , Verdú‐Ricoy, J. , Siliceo, I. , & Salvador, A. (2012). Intraspecific variation of reproductive traits in a mediterranean lizard: clutch, population, and lineage effects. Evolutionary Biology, 39, 106–115. 10.1007/s11692-011-9144-5 [DOI] [Google Scholar]
- Diniz‐Filho, J. A. F. (2008). Bergmann's rule, natural selection and the end of the Panglossian paradigm in ecogeographical analyses. Journal of Biogeography, 35, 577–578. 10.1111/j.1365-2699.2007.01865.x [DOI] [Google Scholar]
- Dunham, A. E. (1978). Food availability as a proximate factor influencing individual growth rates in the iguanid lizard Sceloporus merriami . Ecology, 59, 770–778. 10.2307/1938781 [DOI] [Google Scholar]
- Epova, L. A. , Kuranova, V. N. , Yartsev, V. V. , & Absalyamova, E. N. (2016). Age, body sizes and growth of Zootoca vivipara (Sauria: Lacertidae) from its mountain populations in the Kuznetsk Alatau (southeast of the Western Siberia). Modern Herpetology (Saratov), 16(1/2), 51–60. 10.18500/1814-6090-2016-16-1-2-51-60 [DOI] [Google Scholar]
- Fairbairn, D. J. (1997). Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annual Review of Ecology & Systematics, 28, 659–687. 10.1146/annurev.ecolsys.28.1.659 [DOI] [Google Scholar]
- Fairbairn, D. J. (2005). Allometry for sexual size dimorphism: testing two hypotheses for Rensch's rule in the water strider, Aquarius remigis . The American Naturalist, 166, S69–S84. 10.1086/444600 [DOI] [PubMed] [Google Scholar]
- Fairbairn, D. J. , Blanckenhorn, W. U. , & Szekely, T. (2007). Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford: Oxford University Press; 10.1641/B580514 [DOI] [Google Scholar]
- Falconer, D. S. (1989). Introduction to quantitative genetics (3rd ed.). New York:Wiley. [Google Scholar]
- Ficetola, G. F. , Scali, S. , Denoël, M. , Montanaro, G. , Vukov, T. D. , Zuffi, M. A. L. , & Padoa‐Schioppa, E. (2010). Ecogeographical variation of body size in amphibians: comparing the hypotheses using the newt Triturus carnifex . Global Ecology and Biogeography, 19, 485–495. 10.1111/j.1466-8238.2010.00535.x [DOI] [Google Scholar]
- Foucart, T. , Lourdais, O. , DeNardo, D. , & Heulin, B. (2014). Influence of reproductive mode on metabolic costs of reproduction: insight from the bimodal lizard Zootoca vivipara . Journal of Experimental Biology, 217, 4049–4056. 10.1242/jeb.104315 [DOI] [PubMed] [Google Scholar]
- Freckleton, R. P. (2011). Dealing with collinearity in behavioural and ecological data: model averaging and the problems of measurement error. Behavioural Ecology & Sociobiology, 65, 91–101. 10.1007/s00265-010-1045-6 [DOI] [Google Scholar]
- Gardner, J. L. , Peters, A. , Kearney, M. R. , Joseph, L. , & Heinsohn, R. (2011). Declining body size: a third universal response to warming? Trends in Ecology & Evolution, 26, 285–291. 10.1016/j.tree.2011.03.005 [DOI] [PubMed] [Google Scholar]
- Garel, M. , Solberg, E. J. , Sæther, B. E. , Herfindal, I. , & Høgda, K. A. (2006). The length of growing season and adult sex ratio affect sexual size dimorphism in moose. Ecology, 87, 745–758. [DOI] [PubMed] [Google Scholar]
- Gaston, K. J. , Chown, S. L. , & Evans, K. L. (2008). Ecogeographical rules: elements of a synthesis. Journal of Biogeography, 35, 483–500. 10.1111/j.1365-2699.2007.01772.x [DOI] [Google Scholar]
- Green, D. M. , & Middleton, J. (2013). Body size varies with abundance, not climate, in an amphibian population. Ecography, 36, 947–955. 10.1111/j.1600-0587.2013.00063.x [DOI] [Google Scholar]
- Guillette, L. J. Jr (1982). The evolution of viviparity and placentation in the high elevation, Mexican lizard Sceloporus aeneus . Herpetologica, 38, 94–103. [Google Scholar]
- Herczeg, G. , Gonda, A. , & Merilä, J. (2010). Rensch's rule inverted – Female‐driven gigantism in nine‐spined stickleback Pungitius pungitius . Journal of Animal Ecology, 79, 581–588. 10.1111/j.1365-2656.2010.01665.x [DOI] [PubMed] [Google Scholar]
- Heulin, B. (1985). Démographie d'une population de Lacerta vivipara de basse altitude. Acta Oecologica, Oecologica Generalis, 6, 261–280. [Google Scholar]
- Hjernquist, M. B. , Söderman, F. , Jönsson, K. I. , Herczeg, G. , Laurila, A. , & Merilä, J. (2012). Seasonality determines patterns of growth and age structure over a geographic gradient in an ectothermic vertebrate. Oecologia, 170, 641–649. 10.1007/s00442-012-2338-4 [DOI] [PubMed] [Google Scholar]
- Horváthová, T. , Cooney, C. R. , Fitze, P. S. , Oksanen, T. A. , Jelić, D. , Ghira, I. , … Jandzik, D. (2013). Length of activity season drives geographic variation in body size of a widely distributed lizard. Ecology and Evolution, 3, 1–19. 10.1002/ece3.613 [DOI] [Google Scholar]
- Hu, Y. , Xie, Y. , Zhu, F. , Wang, C. , & Lei, C. (2010). Variation in sexual size dimorphism among populations: testing the differential plasticity hypothesis. Entomologia Experimentalis Et Applicata, 137, 204–209. 10.1111/j.1570-7458.2010.01054.x [DOI] [Google Scholar]
- Jetz, W. , Ashton, K. G. , & La Sorte, F. A. (2009). Phenotypic population divergence in terrestrial vertebrates at macro scales. Ecology Letters, 12, 1137–1146. 10.1111/j.1461-0248.2009.01369.x [DOI] [PubMed] [Google Scholar]
- Körner, C. H. (2000). Why are there global gradients in species richness? Mountains might hold the answer. Trends in Ecology and Evolution, 15, 513–514. 10.1016/S0169-5347(00)02004-8 [DOI] [Google Scholar]
- Kratochvíl, L. , & Frynta, D. (2002). Body size, male combat and the evolution of sexual dimorphism in eublepharid geckos (Squamata: Eublepharidae). Biological Journal of the Linnean Society, 76, 303–314. 10.1111/j.1095-8312.2002.tb02089.x [DOI] [Google Scholar]
- Kuranova, V. N. , Yartsev, V. V. , Kononova, Y. V. , Protopopova, E. V. , Konovalova, S. N. , Ternovoy, V. A. , … Moskvitina, N. S. (2011). Lacertids (Sauria) in natural foci of infections in human‐transmitted ecosystems of the south‐east of West Siberia. The Problems of Herpetology, 129–135 (in Russian). [Google Scholar]
- Laiolo, P. , Illera, J. C. , & Obeso, J. R. (2013). Local climate determines intra‐ and interspecific variation in sexual size dimorphism in mountain grasshopper communities. Journal of Evolutionary Biology, 26, 2171–2183. 10.1111/jeb.12213 [DOI] [PubMed] [Google Scholar]
- Le Galliard, J.‐F. , Marquis, O. , & Massot, M. (2010). Cohort variation, climate effects and population dynamics in a shortlived lizard. Journal of Animal Ecology, 79(6), 1296–1307. 10.1111/j.1365-2656.2010.01732.x [DOI] [PubMed] [Google Scholar]
- Legendre, P. (2013). lmodel2: model II regression. R package version 1.7‐1. Retrieved from http://CRAN.R-project.org/package=lmodel2 [Google Scholar]
- Lindtke, D. , Mayer, W. , & Böhme, W. (2010). Identification of a contact zone between oviparous and viviparous common lizards (Zootoca vivipara) in central Europe: reproductive strategies and natural hybridization. Salamandra, 46, 73–82. [Google Scholar]
- Litzgus, J. D. , DuRant, S. E. , & Mousseau, T. A. (2004). Clinal variation in body and cell size in a widely distributed vertebrate ectotherm. Oecologia, 140, 551–558. 10.1007/s00442-004-1611-6 [DOI] [PubMed] [Google Scholar]
- Litzgus, J. D. , & Smith, S. E. (2010). Geographic variation in sexual size dimorphism in painted turtles (Chrysemys picta). Journal of Herpetology, 44, 320–326. 10.1670/08-333.1 [DOI] [Google Scholar]
- Lorenzon, P. , Clobert, J. , & Massot, M. (2001). The contribution of phenotypic plasticity to adaptation in Lacerta vivipara . Evolution, 55, 392–404. [DOI] [PubMed] [Google Scholar]
- Lovich, J. E. , & Gibbons, J. W. (1992). A review of techniques for quantifying sexual size dimorphism. Growth, Development and Aging, 56, 269–281. [PubMed] [Google Scholar]
- Madsen, T. , & Shine, R. (1993). Phenotypic plasticity in body sizes and sexual size dimorphism in European grass snakes. Evolution, 47, 321–325. 10.1111/j.1558-5646.1993.tb01222.x [DOI] [PubMed] [Google Scholar]
- Madsen, T. , & Shine, R. (1994). Costs of reproduction influence the evolution of sexual size dimorphism in snakes. Evolution, 48, 1389–1397. 10.1111/j.1558-5646.1994.tb05323.x [DOI] [PubMed] [Google Scholar]
- Masaki, S. (1967). Geographic variation and climatic adaptation in a field cricket (Orthoptera: Gryllidae). Evolution, 21, 725–774. 10.1111/j.1558-5646.1967.tb03430.x [DOI] [PubMed] [Google Scholar]
- Meiri, S. , Yom‐Tov, Y. , & Geffen, E. (2007). What determines conformity to Bergmann's rule? Global Ecology and Biogeography, 16, 788–794. 10.1111/j.1466-8238.2007.00330.x [DOI] [Google Scholar]
- Millien, V. , Kathleen, L. S. , Olson, L. , Smith, F. A. , Wilson, A. B. , & Yom‐Tov, Y. (2006). Ecotypic variation in the context of global climate change: revisiting the rules. Ecology Letters, 9, 853–869. 10.1111/j.1461-0248.2006.00928.x [DOI] [PubMed] [Google Scholar]
- Mousseau, T. A. (1997). Ectotherms follow the converse to Bergmann's rule. Evolution, 51, 630–632. 10.1111/j.1558-5646.1997.tb02453.x [DOI] [PubMed] [Google Scholar]
- Orlova, V. F. (1975). Systematics and some ecological and morphological peculiarities of the wood lizards of the genus Lacerta. Ph.D. Diss, Moscow Lomonosov State University; (in Russian). [Google Scholar]
- Oufiero, C. E. , Gartner, G. E. A. , Adolph, S. C. , & Garland, T. Jr (2011). Latitudinal and climatic variation in scale counts and body size in Sceloporus lizards: a phylogenetic perspective. Evolution, 65, 3590–3607. 10.1111/j.1558-5646.2011.01405.x [DOI] [PubMed] [Google Scholar]
- Pearson, D. , Shine, R. , & Williams, A. (2002). Geographic variation in sexual size dimorphism within a single snake species (Morelia spilota, Pythonidae). Oecologia, 131, 418–426. 10.1007/s00442-002-0917-5 [DOI] [PubMed] [Google Scholar]
- Pianka, E. R. , & Vitt, L. J. (2003). Lizards: windows to the evolution of biodiversity. Berkeley, CA: University of California Press. [Google Scholar]
- Pilorge, T. (1987). Density, size structure, and reproductive characteristics of three populations of Lacerta vivipara (Sauria: Lacertidae). Herpetologica, 43, 345–356. [Google Scholar]
- Pilorge, T. , & Xavier, F. (1981). Le lezard vivipare (Lacerta vivipara J.) dans la region du Puy‐de‐Dôme: écologie et stratégie de reproduction. Annales De La Station Biologique De Besse‐en‐Chandesse, 15, 32–59. [Google Scholar]
- Pincheira‐Donoso, D. , Hodgson, D. J. , & Tregenza, T. (2008). The evolution of body size under environmental gradients in ectotherms: why should Bergmann's rule apply to lizards? BMC Evolutionary Biology, 8, 68–78. 10.1186/1471-2148-8-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2018). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Recknagel, H. , Layton, M. , Carey, R. , Leitão, H. , Sutherland, M. , & Elmer, K. R. (2018). Melanism in common lizards (Squamata: Lacertidae: Zootoca vivipara): new evidence for a rare but widespread ancestral polymorphism. Herpetology Notes, 11, 607–612. [Google Scholar]
- Reichling, H. (1957). Transpiration und Vorzugstemperatur Mitteleuropäischer Reptilien und Amphibien. Zoologische Jahrbücher, 67, 1–64. [Google Scholar]
- Richards, S. A. , Whittingham, M. J. , & Stephens, P. A. (2011). Model selection and model averaging in behavioural ecology: the utility of the IT‐AIC framework. Behavioral Ecology and Sociobiology, 65, 77–89. 10.1007/s00265-010-1035-8 [DOI] [Google Scholar]
- Roff, D. A. (2002). Life history evolution. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Roitberg, E. S. (2007). Variation in sexual size dimorphism within a widespread lizard species In Fairbairn D. J., Blanckenhorn W. U., & Szekely T. (Eds.), Sex, size, and gender roles: evolutionary studies of sexual size dimorphism (pp. 143–153, 216–217). Oxford, UK: Oxford University Press; 10.1093/acprof:oso/9780199208784.003.0016 [DOI] [Google Scholar]
- Roitberg, E. S. , Eplanova, G. V. , Kotenko, T. I. , Amat, F. , Carretero, M. A. , Kuranova, V. N. , … Yakovlev, V. A. (2015). Geographic variation of life‐history traits in the sand lizard, Lacerta agilis: testing Darwin's fecundity‐advantage hypothesis. Journal of Evolutionary Biology, 28(3), 613–629. 10.1111/jeb.12594 [DOI] [PubMed] [Google Scholar]
- Roitberg, E. S. , Kuranova, V. N. , Bulakhova, N. A. , Orlova, V. F. , Eplanova, G. V. , Shamgunova, R. R. , … Yakovlev, V. A. (2012). Geographic variation in reproductive traits and female body size in the common lizard, Zootoca vivipara . The Problems of Herpetology, 274–279. Minsk (in Russian). [Google Scholar]
- Roitberg, E. S. , Kuranova, V. N. , Bulakhova, N. A. , Orlova, V. F. , Eplanova, G. V. , Zinenko, O. I. , … Yakovlev, V. A. (2013). Variation of reproductive traits and female body size in the most widely‐ranging reptile species: testing the effects of reproductive mode, lineage, and climate. Evolutionary Biology, 40(3), 420–438. 10.1007/s11692-013-9247-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitberg, E. S. , Orlova, V. F. , Kuranova, V. N. , Bulakhova, N. A. , Zinenko, O. I. , Ljubisavljevic, K. , … Böhme, W. (2011). Inter‐observer and intra‐observer differences in measuring body length: a test in the common lizard, Zootoca vivipara . Amphibia‐Reptilia, 32, 477–484. 10.1163/156853811X601636 [DOI] [Google Scholar]
- Roitberg, E. S. , & Smirina, E. M. (2006). Age, body size and growth of Lacerta agilis boemica and L. strigata (Reptilia, Lacertidae): a comparative study of two closely related lizard species based on skeletochronology. Herpetological Journal, 16, 133–148. [Google Scholar]
- Romano, A. , & Ficetola, G. F. (2010). Ecogeographic variation of body size in the spectacled salamanders (Salamandrina): influence of genetic structure and local factors. Journal of Biogeography, 37, 2358–2370. 10.1111/j.1365-2699.2010.02369.x [DOI] [Google Scholar]
- Rutschmann, A. , Miles, D. B. , Le Galliard, J.‐F. , Richard, M. , Moulherat, S. , Sinervo, B. , & Clobert, J. (2016). Climate and habitat interact to shape the thermal reaction norms of breeding phenology across lizard populations. Journal of Animal Ecology, 85, 457–466. 10.1111/1365-2656.12473 [DOI] [PubMed] [Google Scholar]
- Rypel, A. L. (2014). Cold‐water connection: Bergmann's rule in North American freshwater fishes. The American Naturalist, 183, 147–156. 10.1086/674094 [DOI] [PubMed] [Google Scholar]
- Saino, N. , & De Bernardi, F. (1994). Geographic variation in size and sexual dimorphism across a hybrid zone between carrion crows Corvus corone corone and hooded crows C. c. cornix . Canadian Journal of Zoology, 72, 1543–1550. 10.1139/z94-205 [DOI] [Google Scholar]
- Schielzeth, H. (2010). Simple means to improve the interpretability of regression coefficients. Methods in Ecology & Evolution, 1, 103–113. 10.1111/j.2041-210X.2010.00012.x [DOI] [Google Scholar]
- Sears, M. W. (2005). Geographic variation in the life history of the sagebrush lizard: the role of thermal constraints on activity. Oecologia, 143, 25–36. 10.1007/s00442-004-1767-0 [DOI] [PubMed] [Google Scholar]
- Sears, M. W. , & Angilletta, M. J. (2004). Body size clines in Sceloporus lizards: proximate mechanisms and demographic constraints. Integrative and Comparative Biology, 44, 433–442. 10.1093/icb/44.6.433 [DOI] [PubMed] [Google Scholar]
- Shine, R. (1994). Sexual size dimorphism in snakes revisited. Copeia, 1994, 326–346. 10.2307/1446982 [DOI] [Google Scholar]
- Shine, R. (2005). Life‐history evolution in reptiles. Annual Review of Ecology & Systematics, 36, 23–46. [Google Scholar]
- Sinervo, B. , Heulin, B. , Surget‐Groba, Y. , Clobert, J. , Miles, D. B. , Corl, A. , … Davis, A. (2007). Models of density‐dependent genic selection and a new rock‐paper‐scissors social system. The American Naturalist, 170, 663–680. 10.1086/522092 [DOI] [PubMed] [Google Scholar]
- Smith, R. J. (1999). Statistics of sexual size dimorphism. Journal of Human Evolution, 36, 423–459. 10.1006/jhev.1998.0281 [DOI] [PubMed] [Google Scholar]
- Stamps, J. A. (1993). Sexual size dimorphism in species with asymptotic growth after maturity. Biological Journal of the Linnean Society, 50, 123–145. 10.1006/bijl.1993.1050 [DOI] [Google Scholar]
- Stillwell, R. C. , Morse, G. E. , & Fox, C. W. (2007). Geographic variation in body size and sexual size dimorphism of a seed‐feeding beetle. The American Naturalist, 170, 358–369. 10.1086/520118 [DOI] [PubMed] [Google Scholar]
- Strijbosch, H. , & Creemers, R. C. M. (1988). Comparative demography of sympatric populations of Lacerta vivipara and Lacerta agilis . Oecologia, 76, 20–26. 10.1007/BF00379595 [DOI] [PubMed] [Google Scholar]
- Surget‐Groba, Y. , Heulin, B. , Guillaume, C. P. , Puky, M. , Semenov, D. , Orlova, V. , … Smajda, B. (2006). Multiple origins of viviparity, or reversal from viviparity to oviparity? The European common lizard (Zootoca vivipara, Lacertidae) and the evolution of parity. Biological Journal of the Linnean Society, 87, 1–11. 10.1111/j.1095-8312.2006.00552.x [DOI] [Google Scholar]
- Taylor, H. L. (2003). Phenotypic and reproductive responses of Aspidoscelis tigris (Squamata: Teiidae) to shifts in winter precipitation across the southern Sierra Nevada range, Kern County, California. The Southwestern Naturalist, 48, 680–684. [DOI] [Google Scholar]
- Teplitsky, C. , & Millien, V. (2014). Climate warming and Bergmann's rule through time: is there any evidence? Evolutionary Applications, 7, 156–168. 10.1111/eva.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe, R. S. , & Baez, M. (1987). Geographic variation within an island: univariate and multivariate contouring of scalation, size, and shape of the lizard Gallotia galloti . Evolution, 41, 256–268. 10.1111/j.1558-5646.1987.tb05795.x [DOI] [PubMed] [Google Scholar]
- Uller, T. , & Olsson, M. (2003). Life in the land of the midnight sun: are northern lizards adapted to longer days? Oikos, 101, 317–322. 10.1034/j.1600-0706.2003.12374.x [DOI] [Google Scholar]
- Vervust, B. , Van Dongen, S. , & Van Damme, R. (2009). The effect of preservation on lizard morphometrics – An experimental study. Amphibia‐Reptilia, 30, 321–329. 10.1163/156853809788795209 [DOI] [Google Scholar]
- Watkins, G. C. (1996). Proximate causes of sexual size dimorphism in the iguanian lizard Microlophus occipitalis . Ecology, 77, 1473–1482. 10.2307/2265544 [DOI] [Google Scholar]
- Werner, E. E. , & Anholt, B. R. (1993). Ecological consequences of the trade‐off between growth and mortality rates mediated by foraging activity. The American Naturalist, 142, 242–272. 10.1086/285537 [DOI] [PubMed] [Google Scholar]
- Zamudio, K. R. (1998). The evolution of female‐biased sexual size dimorphism: a population‐level comparative study in horned lizards (Phrynosoma). Evolution, 52, 1821–1833. 10.1111/j.1558-5646.1998.tb02259.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original individual‐based data on body size (SVL), as well as the geographic and climatic data, are archived into the public repository Dryad https://doi.org/10.5061/dryad.r7sqv9s7v.


