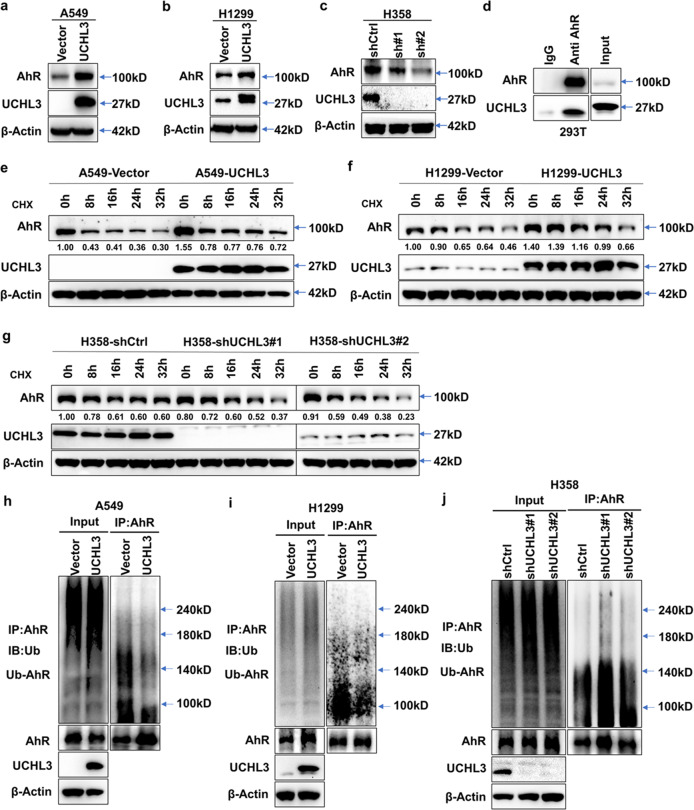
Fig. 4.
UCHL3 interacts with AhR and stabilizes the AhR protein through deubiquitination. a–c Western blot analysis was used to detect the expression level of AhR in A549 (a), H1299 (b) and H358 (c) cells after overexpression or depletion of UCHL3. d Exogenous UCHL3 and AhR proteins interacted in HEK293T cells. AhR and UCHL3 were coexpressed in HEK293T cells, and the AhR protein was immunoprecipitated with anti-AhR antibody. IgG served as a negative control, and exogenous UCHL3 was detected by WB. e, f UCHL3 overexpression delayed AhR protein degradation. After the treatment of UCHL3-overexpressing A549 (e) and H1299 (f) cells with cycloheximide (CHX, 10 μg/ml) for the indicated durations, AhR protein expression was analyzed by WB. Quantification of the AhR protein band was performed using ImageJ software. g UCHL3 knockdown enhanced AhR protein degradation. After UCHL3 knockdown, H358 cells were treated with cycloheximide (CHX, 10 μg/ml) for the indicated duration, and AhR protein expression was analyzed by WB. Quantification of the AhR protein band was performed using ImageJ software. h, i The lysates of A549 (h) and H1299 (i) cells stably overexpressing UCHL3 and vector-transfected cells containing 1 mg of total protein for each panel were immunoprecipitated with 2 μg of anti-AhR antibody, following which AhR ubiquitination was examined using anti-Ub antibody. j The lysates of stable UCHL3-knockdown H358 cells and shCtrl-transfected cells containing 1 mg of total protein were immunoprecipitated with 2 μg of anti-AhR antibody, and AhR ubiquitination was examined using anti-Ub antibody