Abstract
Several multidrug-resistant organisms have emerged, which increases the threat to public health around the world and a limited number of therapeutics were available to counteract these issues. Thus, researchers are trying to find out novel antimicrobials to overcome multidrug-resistant issues. The present study aimed to isolate antibacterial principles against the clinical isolates of multidrug-resistant (MDR) Staphylococcus aureus from the ethyl acetate extract of Gycosmis pentaphylla. The isolation and structural characterization of bioactive compounds were carried out using various chromatographic techniques (TLC, column, HPLC, and LC-MS) and spectral studies such as FT-IR, CHNS analysis, 1H-NMR, and 13C-NMR. The antimicrobial potential of isolated compounds was assessed according to the standard methods. The isolated compounds were identified as arborine and skimmianine, which exhibited a significant antibacterial effect with the lowest MIC and MBC values against MDR S. aureus and in vitro kinetic and protein leakage assays supported the antimicrobial activity. Significant morphological changes such as uneven cell surfaces and morphology, cell shrinkage, and cell membrane damages were observed in the MDR S. aureus upon the treatment of arborine and skimmianine. The present investigation concludes that the isolated arborine and skimmianine compounds from G. pentaphylla harbor a strong antibacterial activity against MDR S. aureus and may be used as alternative natural drugs in the treatment of MDR S. aureus.
Keywords: Gycosmis pentaphylla, antibacterial compounds, chromatography, spectral study, MDR Staphylococcus aureus, arborine, skinmmianine
Introduction
Staphylococcus aureus is a Gram-positive bacterium that belongs to the family Staphylococaceae and is often found at a commensal on the skin, skin glands, and mucous membranes, particularly in the nose of a healthy individual (1). It is causing infections ranging from relatively mild skin and soft tissue infections to life-threatening sepsis, pneumonia, osteomyelitis, endocarditis, as well as toxin-mediated syndromes such as toxic shock syndrome and food poisoning (2). Multidrug resistant S. aureus (MRSA) is resistant to two or more antimicrobial agents like penicillin, oxacillin, ampicillin, and methicillin. In general, MRSA infections have been categorized into four groups based on their sources: healthcare-associated hospital-onset MRSA (HAHO-MRSA), community-associated MRSA (CA-MRSA), healthcare-associated MRSA with community-onset (HACO-MRSA), and livestock-associated MRSA (LA-MRSA) (3). Although MRSA infections mainly occur in hospitals, the human illness caused by community-associated MRSA (CA-MRSA) is increasing considerably (4).
In recent years, drug-resistant human pathogenic bacteria have been frequently reported (5). The drug-resistant Enterotoxigenic Escherichia coli pathogenic bacteria demand the development of new antimicrobial drugs with a novel mode of action, targeting either the cell membrane or intracellular targets (6). Increasing the bacterial resistance is prompting a resurgence in the research on the antimicrobial role of herbs available in nature as effective combinations against antibiotics-resistant bacteria (7). Moreover, using natural products also help to diminish the toxicity of the drugs when they are used on humans (8).
Since ancient times, plants have been the primary sources of many therapeutic agents that possess several secondary metabolites with significant physiological effects. Recently, many active ingredients have been isolated from plants and those are used to develop synthetic analogs for the treatment of ailments. There are 300 plant species frequently used in 7,800 drug-manufacturing units around India, which consume more than 2,000 tons of herbs annually (9). Generally, bioactive principles are isolated from different parts of plants such as leaves, bark, roots, seeds, etc. based on folklore usage. According to WHO, medicinal plants would be the best source to obtain a variety of drugs (10), and ~20% of the known plants were subjected to biological tests and a sustainable number of new antibiotics were introduced into the market (11). Concurrently, the characterizations of antimicrobial compounds from medicinal plants have been very challenging to the researchers (12). Hence, a systemic screening of plants for the identification of antimicrobial compounds to act against microbial pathogens needs an hour. Recently, many researchers have been focused on the isolation of potential bioactive compounds from medicinal plants to produce high-quality and potential secondary metabolites responsible for the control of microorganisms (13, 14). With this background, the present study was aimed to isolate antibacterial principles from Glycosmis pentaphylla.
Materials and Methods
Isolation and Identification of MDR S. aureus
About 500 specimens [wound (138), pus (122), blood (119), sputum (70), and urine (51)] were collected from patients suspected to have S. aureus infections from private and government hospitals in and around Salem and Namakkal Districts, Tamil Nadu, India. The present study has ethical clearance from the institutional ethical committee (reference no.: PU/IEC/HR/2014/008 dated: 31/06/2014), Periyar University, Salem, Tamil Nadu. The methods of isolation and identification of the selected MDR S. aureus strains were described in our previous published data (15, 16). The isolation and identification of MDR S. aureus were carried out by the standard methods such as colony morphology on differential media, microscopic observations, and biochemical tests, and confirmed by molecular analysis like 16s rRNA sequencing. The antibiotic-resistant potential of isolated S. aureus was identified using the standard antimicrobial susceptibility test and identification of antibiotic-resistant marker genes such as MecA, blaZ, Aph (III), etc. The 16s rRNA sequence of isolated MDR S. aureus (101, 270, 315, 319, and 410) strains used in the present study is deposited in GenBank and GenBank accession nos. are KU198419 (S. aureus 101), KX290715.1 (S. aureus 270), KX454514 (S. aureus 315), KX447584 (S. aureus 319), and KX447585 (S. aureus 410).
Plant Material and Extraction
The healthy and young leaves of G. pentaphylla (Figure 1) were collected from Vellimalai village (Latitude 11° 47′ 55.6836″ N, longitude 78° 41′ 58.0056″ E), Villupuram district, Tamil Nadu, India. The taxonomic identification of collected plant material was done by the Botanical Survey of India (Southern Circle), Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India (reference letter No BSI/SRC/5/26/2016/402). The voucher specimen of collected plant material was deposited (No. PU/BT/NDRL/2016/09) in the Natural Drug Research Laboratory, Department of Biotechnology, Periyar University, Salem, Tamil Nadu, India. The collected plant materials were washed twice in running tap water and shade dried at room temperature for 3 weeks. The air-dried plant leaves were pulverized, using an electric blender to make a fine powder. A total of 3 kg of powdered G. pentaphylla leaves was sequentially extracted with different organic solvents (1:10 solvent ratio) in an increasing polarity (hexane, chloroform, ethyl acetate, acetone, and methanol) using a Soxhlet apparatus until the efflux solvents become colorless. All extracts were filtered through filter paper (Whatman No. 1) and dried under vacuum at 40°C. The dried crude solvent extracts were stored in a freezer at −4°C for further study (17).
Figure 1.

A close view of Glycosmis pentaphylla (Rutaceae).
Isolation of Antibacterial Principles
The preliminary antibacterial activity results of various crude extracts of G. pentaphylla (15) revealed that the crude ethyl acetate of G. pentaphylla possesses a significant high antibacterial potential; thus, the ethyl acetate extract was selected for isolation of active principles. The selected ethyl acetate extract was subjected to different chromatography techniques and the structure of isolated bioactive compounds were identified using various spectral studies.
Separation of Active Compounds by Chromatography
The ethyl acetate extracts (in both low and high concentration) were spotted on the TLC plate (TLC Silica gel F254, Merck, Germany, 10 × 10 cm2) origin and kept in a TLC chamber containing the desired solvent system. The solvent traveled to the top of the TLC plate by capillary action until it reached the solvent front. The TLC plate was taken out of the solvent tank. The dried TLC plate was viewed by iodine vapor and visualized under UV light (low and high wavelength). The same method was followed to find the optimal solvent system with other TLC plates in various solvent systems like hexane:ethyl acetate, ethyl acetate:methanol, chloroform:ethyl acetate, and chloroform:methanol in 100:0 to 0:100 ratios by increasing 10% more polar solvents used in the solvent system until a good resolution was noticed (18). The selected bioactive extract of G. pentaphylla was subjected to column chromatography to obtain fractions by increasing polarity of eluents using different solvent systems (chosen from the TLC trials) of chloroform:methanol 100:0, 80:20, 70:30, 60:40, and 50:50 ratios by increasing 10–20% more polar solvent used in the solvent system. The column (120 × 4 cm) was packed with a solution of silica gel (60–120 mesh) with ethyl acetate using the wet slurry method. This involves preparing a solution of silica gel, with chloroform filled in the column up to one-third (about 40 cm) length. A significant amount of chloroform was added to the column and allowed to drain for silica gel setting in the column and the volume of the solvent collected was again poured back into the column.
The ethyl acetate extract (60 g) was absorbed into silica gel (100–200 mesh) by triturating in a mortar and left for 1 h to dry. The dried plant extract coated with silica gel was loaded on the prepared column by adding gently into the column filled with chloroform without any air bubbles and eluted with the desire solvent system. A total of 60 fractions was obtained and tested for their antibacterial potential against MDR S. aureus strains (clinical isolates) according to the method of Srinivasan et al. (19). The G. pentaphylla leaf ethyl acetate extract 9.7:0.3 chloroform:methanol ratio fraction (GPLCM-58F) showed significant antibacterial activity against S. aureus strains, which selected for further purification of bioactive compounds. The selected fraction was again monitored by TLC (pre-coated plate, 0.02 mm thick) to determine the optimal solvent system for further purification in column chromatography. Another small size column chromatography (60 × 2 cm) was employed for further purification of selected fractions as per the abovementioned procedures. The fractions were eluted with chloroform:methanol (9:1–9.8:0.2 ratio) solvent system by increasing 0.1% of the volume of the polar solvents used in the solvent system. All the collected column fractions were again examined for antimicrobial activity and purity by TLC (pre-coated plate, 0.02 mm thick) for the single spot and the Rf values were calculated.
A total of two fractions (GPSC-55SF and GPSC-73SF) exhibited considerable antibacterial activity; these fractions again were subjected to preparative TLC purification (20). The preparative TLC plate was performed based on the noted Rf values. The fractions were scraped with silica powder using the sterile scoop and the collected UV-active pure compounds were dissolved in ethyl acetate solvent, and it can allow stirring with a magnetic stirrer for 1 h. After the pure fraction of active compounds was separated in the solvent, it was collected and concentrated under vacuum conditions. The UV-active pure fractions of the compound (GP-1 and GP-2) with similar Rf value was pooled together and subjected to the antimicrobial activity and spectral studies.
Structural Identification of Isolated Compounds
Liquid Chromatography–Mass Spectroscopy (LC-MS) Analysis
LC-MS analyses of the two isolated compounds were carried out according to the method of Natarajan et al. (21) using a Thermo/Finnigan Surveyor System consisting of a degasser, binary pump, autosampler, and column heater. The LC was coupled with the Ion Trap mass spectrometer (Thermofleet, LCQ-Fleet) supported with an ESI ion source. Data acquisition and mass spectrometric evaluation were carried out in a personal computer with Data Analysis software (Qual Browser; Thermo Electron, San Jose, CA). For the chromatographic separation, Acquity BEC 1.7-μm C18 column (2.1 × 50 mm) was used. The column was programmed to run 95% of 0.1% acetic acid in water and 5% acetonitrile for both compounds 1 and 2. The final, elution program was operated at the linear gradient level of acetonitrile from 100 to 5% for a minimum 2 min. The flow rate was 1 ml/min and the injection volume was 1 μl. The capillary voltage, column temperature, nebulizer pressure, and gas flow rate were set to 20 V, 300°C, 40 psi, and 15 ml/min, respectively, for the entire MS analysis.
Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
The structures of the isolated compounds were elucidated primarily by homonuclear (1D) NMR (22). 1D NMR experiments included 1H and 13C NMR, which used to locate atom positions and fragment units. NMR spectra were recorded on a Bruker Avance 300-MHz and/or Bruker Avance 600-MHz spectrometers coupled with Topsin acquisition software. Samples were dissolved in 500–600 μl of suitable deuterated solvent. An NMR experiment on samples with very little mass was carried out using Shigemi NMR tubes (100–200 μl sample). Signals were recorded in chemical shifts (δ) and expressed in parts per million (ppm), with coupling constants (J) calculated in hertz (Hz).
Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
The UV-active pure compounds (GP-1 and GP-2) of fraction samples were used for FT-IR analysis. The dried compounds (each 3 mg) were encapsulated in 100 mg of KBr pellet, to prepare translucent sample discs. The compounds were loaded in an FTIR spectroscope (Shimadzu, Japan), measured from 400 to 4,000 cm−1 and at a resolution of 4 cm−1.
High-Performance Liquid Chromatography (HPLC) Analysis
The HPLC analysis of active compounds isolated from G. pentaphylla ethyl acetate extract was performed using the modified method of Hawry et al. (23). About 1 mg of concentrated sample was dissolved in 1 ml of chloroform:methanol (9.8:0.2) and 20 μl was injected to determine the purity of compounds.
Antibacterial Activity of Isolated Compounds
The selected MDR S. aureus strain suspension culture was prepared by growing a single colony overnight in Luria-Bertani (LB) broth with a turbidity of 0.5 McFarland standards. The antibacterial activity of isolated compounds was evaluated against MDR S. aureus strains using the agar well diffusion method (24). The isolated compounds (10 μg/ml) were added into the corresponding wells using a micropipette. Commercial antibiotic (amoxicillin) was used as a positive control, whereas DMSO served as a negative control. The plates were incubated at 37°C for 24 h and the diameter of the growth inhibition zone was measured.
MIC and MBC Test
Minimum inhibitory concentration (MIC) of the isolated compounds was carried out using the microdilution method (25), using LB broth (Hi-media, India), and inoculum was adjusted to 2.5 × 105 CFU/ml. In brief, 10 μl (2.5 × 105 CFU/ml) of each MDR S. aureus strains was added individually in 1 ml of LB broth. Different concentrations (0.5, 1, and 2 μg/ml) of the isolated compounds were mixed with test tubes containing the MDR S. aureus strains. After 24-h incubation, the MIC values were obtained by visual observation of bacterial growth. The minimum bactericidal concentration (MBC) value of the isolated compounds was evaluated using the method of Natarajan et al. (25). The MBC values were determined by sub-culturing (10 μl) the MIC dilutions into the sterile Müller Hinton agar plates and incubated at 37°C for 24 h, and the results were observed.
Mechanism of Action of Isolated Compounds Against MDR S. aureus
Protein Leakage Assay
The impact of the isolated compounds on the MDR S. aureus cells was measured in the terms of leakage of intracellular protein materials. The MDR S. aureus were treated with the isolated compounds at 37°C for 120 min; each cell suspension was centrifuged at 7,000 RPM. About 100 μl of each sample supernatant was mixed with 900 μl of Bradford reagent and then incubated for 10 min. The optical density was measured at 595 nm. Bovine serum albumin was used as a standard protein and the experiment was done in triplicate (26).
In vitro Killing Kinetic Assay
The time-kill kinetic assays were performed in five test tubes (two sets) containing an initial inoculum of 1 × 106 CFU/ml in tryptic soy broth with isolated compounds according to the modified method of García et al. (27). Changes in the bacterial count during exposure of the isolated compounds to the bacteria were monitored in five test tubes. MDR S. aureus culture alone served as a control. The bacterial counts of the treated samples were determined in 3-h intervals up to 12-h periods (0, 3, 6, 9, and 12 h) of incubation. One hundred microliters of treated samples was diluted and 10 μl of each sample was spread on Baird–Parker agar plates and incubated at 37°C overnight, and the control samples were incubated under the same conditions. The number of viable cells in each tube was estimated after counting bacterial colonies on plates and by multiplying the appropriate dilution factor (28). All the experiments were conducted in triplicate, and mean values were measured.
SEM Analysis
The effect of isolated compounds on the morphology of MDR S. aureus strains was observed under scanning electron microscopy (SEM) after the bacterial cells were treated with the isolated compounds (arborine and skimmianine) for 6 h. After the treatment, 1 ml of each test bacterial strain was collected, centrifuged, and washed three times with phosphate buffer saline and incubated for 30 min at 4°C and fixed with 2.5% glutaraldehyde. The MDR S. aureus cells were dehydrated in ethanol, freeze-dried under vacuum condition, coated with an ion sputtering apparatus, and observed through SEM (Hitachi S-3400N). All strains of MDR S. aureus cells that are not exposed to the isolated compounds and a standard reference strain of S. aureus (MTCC 96) [procured from Microbial Type Culture Collection and Gene Bank (MTCC), Institute of Microbial Technology, Chandigarh, India] cells were similarly processed and used as controls (29).
Results
Isolation of Bioactive Compounds
Chloroform and methanol were used in various proportions as mobile phases. After elution, the purity of each fraction was tested by analytical TLC using chloroform:methanol in the ratio 9.7:0.3, which showed clear separation of fractions. The TLC containing nine major active compounds with different Rf values and similar Rf value fractions were scraped and pooled together (Figure S1) for further analysis. The ethyl acetate solvent extract of G. pentaphylla leaves (60 g) was applied to a silica gel column for isolation of the bioactive compounds. The column was eluted with a linear gradient solvent system consisting of chloroform:methanol, by increasing 10% polarity (100:0 to 0:100) of the solvents and the fractions were collected in a glass container. The similar Rf value fractions were pooled together according to the TLC profile and serially numbered (GP1, GP2, GP3, etc.) and all those fractions were kept at room temperature to allow condensation. After solvent evaporation, all collected fractions were tested for their antibacterial activity against the MDR S. aureus strains.
Spectroscopic Analysis
The structural characterization of the isolated compounds was identified using NMR, LC-MS mass spectrometry, and HPLC analysis. UV and IR spectroscopy along with the determination melting point was carried out as they required for both the physical and structural characterization of isolated compounds.
HPLC Analysis
The purity of isolated compounds was checked by HPLC analysis. The isolated active compound shows a separation peak at a retention time of 3.212 and 3.434 min for active compounds 1 and 2 respectively. In both the solvent system used, the purity of the active compounds was indicated as a single sharp peak (Figure S2).
Structural Elucidation of Bioactive Compounds
Physical Properties of Isolated Compounds
Compound 1 (arborine) was a yellowish-green color and soluble in ethyl acetate, methyl chloride, and DMSO, and insoluble in water. The melting point of the compound was 160–161°C and the yield was 120 mg. The Rf value of compound 1 was 0.98 mm in analytical TLC using a chloroform:methanol 9.8:0.2 solvent system as the mobile phase, whereas compound 2 (skimmianine) appeared as yellow color powder, soluble in ethyl acetate, methyl chloride, and DMSO. The yield of the isolated compound was 150 mg and the melting point was 180°C. The Rf value of compound 2 was 0.5 mm in analytical TLC using a chloroform:methanol 9.8:0.2 solvent system as the mobile phase.
FT-IR Spectrum Analysis of Compounds
The FT-IR spectrum of arborine shows a broad peak at 1,602 cm−1, which corresponds to the carbonyl group. The aromatic C–H stretching peak was observed at 2,925 cm−1 while aliphatic C–H stretching frequency was found at 2,853 cm−1. The absorption band at 1,264 and 1,402 cm−1 corresponds to the C=N stretch of amide and CH3 bend, respectively. The aromatic C–H out of the plane bending vibration was detected at 765 cm−1 (Figure S3, Table 1). The FT-IR analysis of skimmianine showed an absorption band at 1,616 cm−1 due to the C=N group and characteristic phenyl ether C–O stretching vibration observed at 1,266 and 1,056 cm−1. The C–H stretching vibration corresponding to aromatic was observed at 3,116 cm−1 while aliphatic C–H stretching vibration was identified at 2,937 and 2,839 cm−1. The characteristic methyl bend and aromatic C–H bend out of plane vibrations were seen at 1,390 cm−1 and 739 cm−1, respectively. The stretching frequency of C–O in the furan ring was observed at 1,056 and 1,192 cm−1 (Figure S7, Table 1).
Table 1.
FT-IR spectrum of arborine and skimmianine compounds.
| Compound name | νmax (cm−1) | Functional groups |
|---|---|---|
| Arborine | 2,925 | Aromatic C–H Stretch |
| 2,855 | Aliphatic C–H Stretch | |
| 1,602 | C=O | |
| 1,264 | C–N Stretch | |
| 1,402 | Methyl bend | |
| 765 | Aromatic C–H bend out of the plane | |
| Skimmianine | 3,116 | Aromatic C–H Stretch |
| 2,937, 2,839 | Aliphatic C–H Stretch | |
| 1,616 | C=N Stretch | |
| 1,266, 1,056 | Phenyl ether C–O | |
| 1,390 | Methyl bend | |
| 1,056, 1,192 | Furan C–O | |
| 736 | C–H bend out of the plane |
LC-MS and Elemental Analysis of Compounds
The mass spectra of arborine showed a molecular ion peak at m/z 251 (M+1)+ corresponding to the molecular formula C16H14N2O. The molecular weight was exactly matching with expected structure arborine (Figure S6). The result of the CHNS/O analysis of arborine showed carbon 76.22%, hydrogen 5.48%, nitrogen 10.96%, and oxygen 6.39%, which is in agreement with theoretical values (Table 2). The mass spectra of skimmianine showed a molecular ion peak at m/z 260 (M+1)+ corresponding to the molecular formula C14H13NO4. The molecular weight of the compound exactly matched the expected structure of skimmianine (Figure S10). The result of the CHNS/O analysis of skimmianine showed carbon 63.36%, hydrogen 4.98%, nitrogen 5.02%, and oxygen 24.96%, which is in agreement with theoretical values. The elemental analysis result was interpreted with molecular mass, which revealed the molecular formula of the GP-2 compound as C14H13NO4 (Table 2).
Table 2.
Elemental analysis of arborine and skimmianine compounds.
| Compound name | Elements | Theoretical value (%) | Observed value (%) |
|---|---|---|---|
| Arborine | Carbon | 76.78 | 76.22 |
| Hydrogen | 5.67 | 5.48 | |
| Nitrogen | 11.19 | 10.96 | |
| Oxygen | 6.39 | 6.23 | |
| Skimmianine | Carbon | 64.86 | 63.36 |
| Hydrogen | 5.05 | 4.98 | |
| Nitrogen | 5.40 | 5.02 | |
| Oxygen | 24.68 | 24.96 |
1H-NMR and 13C-NMR Analysis of Compounds
The 1H-NMR spectrum of arborine revealed a total of 14 protons present in the compound. The characteristic N-methyl proton was observed at δ 3.63 ppm as a singlet. The benzylic protons present in the arborine was seen at δ 4.29 ppm as singlet (Figure S4). In the downfield aromatic region, eight aromatic protons were observed between δ 7.28 and δ 7.36 ppm as a multiplet. The quinazoline fused aromatic ring protons numbered 3, 1, and 4 were observed at δ 7.46 ppm (triplet), δ 7.71 ppm (doublet), and δ 8.37 ppm (doublet), respectively (Figure S4, Table 3).
Table 3.
NMR (1H-NMR and 13C-NMR) spectral data of arborine compound.
| Chemical shift (δ ppm) | Proton/carbon numbered | Splitting pattern | Nature of the proton/carbon |
|---|---|---|---|
| 1H-NMR | |||
| 3.63 | 5 | Singlet | N-methyl proton |
| 4.29 | 6 | Singlet | Benzylic proton |
| 7.28–7.36 | 7, 8, 9, 10, 11, 12, 13, 14 | Multiplet | Aromatic (benzene ring) |
| 7.46 | 2, 3 | Triplet | Aromatic (quinazoline ring) |
| 7.71 | 1 | Doublet | Aromatic (quinazoline ring) |
| 8.37 | 4 | Doublet | Aromatic (quinazoline ring) |
| 13C-NMR | |||
| 34.8 | 7 | Singlet | N-methyl carbon |
| 43.4 | 10 | Singlet | Benzylic carbon |
| 114.4 | 1 | Singlet | Aromatic (Quinazoline ring) |
| 120.0 | 8 | Singlet | Aromatic (Quinazoline ring) |
| 125.9 | 3 | Singlet | Aromatic (Quinazoline ring) |
| 127.3 | 14 | Singlet | Aromatic (Benzene ring) |
| 128.2 | 13, 15 | Singlet | Aromatic (Benzene ring) |
| 128.5 | 4 | Singlet | Aromatic (Quinazoline ring) |
| 129.0 | 12, 16 | Singlet | Aromatic (Benzene ring) |
| 133.7 | 2 | Singlet | Aromatic (Quinazoline ring) |
| 134.5 | 11 | Singlet | Aromatic (Benzene ring) |
| 141.4 | 9 | Singlet | Aromatic (Quinazoline ring) |
| 162.2 | 6 | Singlet | C=N (Quinazoline ring) |
| 169.0 | 5 | Singlet | Amide C=O |
In the 13C-NMR of arborine, N-methyl and benzylic carbons are observed at δ 34.8 and δ 43.4 ppm, respectively. The highest carbon chemical shift δ 169.0 ppm was observed for amide carbonyl carbon. The chemical shift for imine carbon (C=N) was seen at δ 162.2 ppm. The aromatic carbon chemical shifts were observed between δ 114.4 and δ 141.4 ppm (Figure S5, Table 3). Three methoxy groups present in skimmianine showed chemical shift values between δ 4.03 and δ 4.49 ppm. All the methoxy protons in the upfield showed a clear singlet pattern. The two protons correspond to the furan ring observed at δ 7.02 and δ 7.56 ppm, respectively, and those protons show a doublet splitting pattern. The other aromatic protons are seen at δ 7.23 and δ 7.99 ppm, which also showed a doublet splitting pattern (Figure S8, Table 4). In the 13C-NMR of skimmianine, the characteristic three methoxy carbons were seen at δ 56.7, δ 58.8, and δ 61.5 ppm. The highest carbon chemical shift δ 164.2 ppm was observed for the methoxy group-attached carbon, which is present in the pyridine ring part of skimmianine. The other methoxy-attached carbons are seen at δ 152.0 and δ 157.0 ppm. The characteristic imine carbon C=N was observed at δ 142.1 ppm. All aromatic magnetically non-equivalent carbons are observed between δ 101.9 and δ 142.8 ppm (Figure S9, Table 4).
Table 4.
NMR (1H-NMR and 13C-NMR) spectral data of skimmianine compound.
| Chemical shift (δ ppm) | Proton/carbon numbered | Splitting pattern | Nature of the proton/carbon |
|---|---|---|---|
| 1H-NMR | |||
| 4.03 | 3 | Singlet | Methoxy |
| 4.11 | 6 | Singlet | Methoxy |
| 4.41 | 7 | Singlet | Methoxy |
| 7.02 | 2 | Doublet | C–H (Furan ring) |
| 7.23 | 5 | Doublet | C–H (Aromatic ring) |
| 7.56 | 1 | Doublet | C–H (Furan ring) |
| 7.99 | 4 | Doublet | C–H (Aromatic ring) |
| 13C-NMR | |||
| 56.7 | 8 | Singlet | Methoxy carbon |
| 58.8 | 9 | Singlet | Methoxy carbon |
| 61.5 | 10 | Singlet | Methoxy carbon |
| 101.9 | 11 | Singlet | Aromatic-fused ring carbon |
| 104.4 | 2 | Singlet | Furan-fused ring carbon |
| 112.2 | 4 | Singlet | Aromatic ring carbon |
| 114.8 | 13 | Singlet | Aromatic-fused ring carbon |
| 117.9 | 5 | Singlet | Aromatic ring carbon |
| 141.4 | 14 | Singlet | Aromatic-fused ring carbon |
| 142.1 | 12 | Singlet | Furan-fused ring carbon |
| 142.8 | 1 | Singlet | Furan ring carbon |
| 152.0 | 6 | Singlet | Aromatic ring carbon |
| 157.0 | 7 | Singlet | Aromatic ring carbon |
| 164.2 | 3 | Singlet | Aromatic ring carbon |
Antibacterial Activity of Isolated Compounds
Arborine and skimmianine compounds showed significant antibacterial activity against clinical isolated MDR S. aureus strains reported that 25 mm to 28mm of growth inhibition zones (Table 5). The results of the antibacterial activity of arborine and skimmianine clearly showed a dose-dependent effect (Table 6). The antibacterial activity results of arborine indicated that MDR S. aureus 101 and 410 strain was highly susceptible to the isolated compounds with the maximum inhibition of growth zone (28 mm) and lower MIC (0.2 μg/ml) and MBC (0.2 μg/ml) values. Similarly, skimmianine compound exhibit the highest antibacterial activity against the MDR S. aureus 315 strain (28 mm) with the lowest MIC (0.2 μg/ml) and MBC (0.2 μg/ml) values. The antibacterial activity was noticed in low concentration with stronger inhibitory potential. The results indicated that arborine and skimmianine exhibit a strong growth inhibition effect on all tested pathogens. The overall results indicated that the arborine and skimmianine possess better antibacterial activity than commercial antibiotics. Even a low MIC and MBC concentration of isolated compounds expressed a remarkable amount of bactericidal activity against the tested MDR S. aureus strains.
Table 5.
Anti-bacterial activity of arborine and skimmianine compounds against MDR S. aureus.
| S. no. | MDR Strains | Arborine | Skimmianine | Amoxicillin | DMSO |
|---|---|---|---|---|---|
| 1 | S. aureus 101 | 28 ± 0.58 | 27 ± 0.24 | 23 ± 0.58 | 00 ± 0.00 |
| 2 | S. aureus 270 | 26 ± 0.00 | 25 ± 0.58 | 26 ± 0.58 | 00 ± 0.00 |
| 3 | S. aureus 315 | 27 ± 0.58 | 28 ± 0.08 | 29 ± 0.58 | 00 ± 0.00 |
| 4 | S. aureus 319 | 25 ± 0.15 | 26 ± 0.73 | 25 ± 0.58 | 00 ± 0.00 |
| 5 | S. aureus 410 | 28 ± 0.39 | 25 ± 0.08 | 27 ± 0.58 | 00 ± 0.00 |
Table 6.
MIC and MBC values of arborine and skimmianine compounds against MDR S. aureus.
| S. no. | MDR strains | MIC and MBC values (μg/ml) | |||||
|---|---|---|---|---|---|---|---|
| Arborine | Skimmianine | Amoxicillin | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | ||
| 1 | S. aureus 101 | 0.2 | 0.2 | 0.2 | 1 | >2 | >2 |
| 2 | S. aureus 270 | 1 | 2 | 2 | >2 | 2 | >2 |
| 3 | S. aureus 315 | 1 | 1 | 0.2 | 0.2 | 1 | 2 |
| 4 | S. aureus 319 | 1 | >2 | 1 | 1 | >2 | >2 |
| 5 | S. aureus 410 | 0.2 | 0.2 | 2 | >2 | 2 | >2 |
Bacterial Mechanism Study of Compounds
Time Kill Kinetic Assay
The time-killing kinetic assay was used to analyze the post-treatment bacterial viability and to define the minimum time required to obtain the bactericidal effect. Both arborine and skimmianine compounds show similar time-killing kinetic patterns of bactericidal effect on the MDR S. aureus strains (Figures 2A,B). Bactericidal activity was gradually increased by the time up to 12 h exposure of the MDR S. aureus strains against arborine and skimmianine compounds at their respective MBC concentration for both the strains and the MDR S. aureus strains were killed within this period. The arborine and skimmianine compounds expressed a time-dependent and prompt bactericidal potential against the tested MDR S. aureus strains, which leads to bacterial death at the early stationary phase, as shown in time-kill curves (Figures 2A,B) compared with the control cells.
Figure 2.
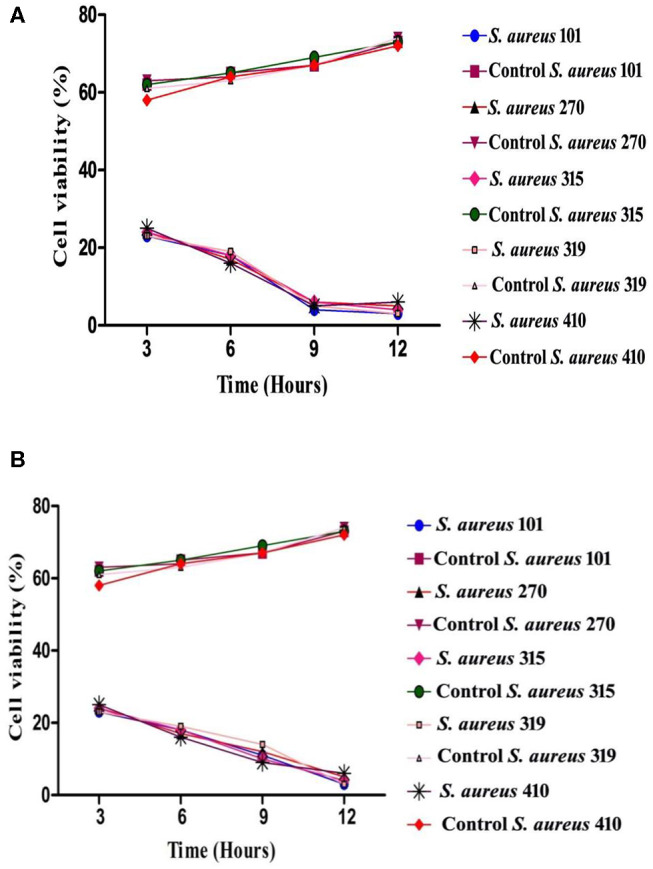
(A) Time-kill kinetic assay of arborine compound on different MDR Staphylococcus aureus strains. (B) Time-kill kinetic assay of skimmianine compound on different MDR Staphylococcus aureus strains.
Protein Leakage Assay
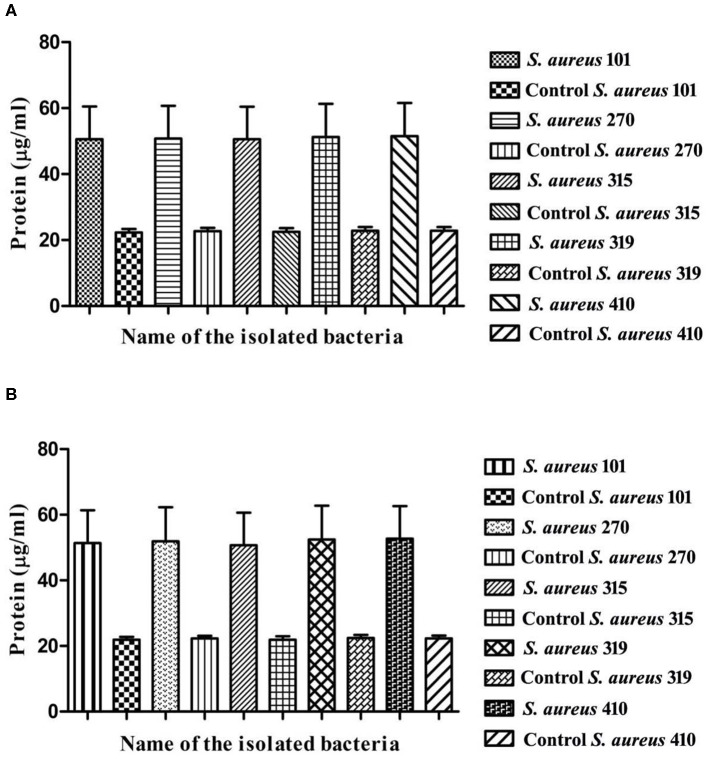
Arborine and skimmianine compounds are known to enhance protein leakage by increasing the membrane permeability in MDR S. aureus strains. To determine the impact of arborine and skimmianine compounds alone on protein leakage, the cells were treated with arborine for 75 μg/ml, resulting in all strains exhibiting 54% protein leakage, and skimmianine compounds were treated for 100 μg/ml and the leakage of proteins was 55%. When the cells were treated with arborine and skimmianine compounds, the amount of protein released from the cells was increased compared to control (commercial antibiotics with recommended doses) (Figures 3A,B).
Figure 3.
(A) Protein leakage assay of arborine compound on different MDR Staphylococcus aureus strains. (B) Protein leakage assay of skimmianine compound on different MDR Staphylococcus aureus strains.
Morphological Changes of MDR S. aureus
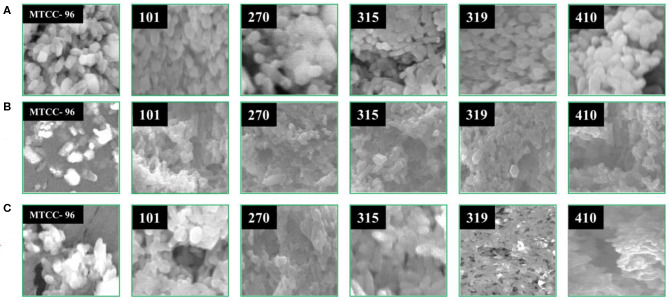
In order to have a better understanding of the antibacterial mechanism, arborine and skimmianine compounds were further studied for their effects on bacterial cell surfaces. Using SEM analysis, the present study observed the morphological changes in all MDR S. aureus strains and the reference standard S. aureus MTCC-96 strain, upon treatment with the antibacterial effect of arborine and skimmianine compounds (Figure 4). In MDR S. aureus, the untreated bacterial cells (control strains) appeared as grape-shaped (cocci), and the surfaces of the cells were intact with no damage observed. The S. aureus cells treated with arborine and skimmianine compounds, was not able to maintain the cocci (grape) shaped characteristics. Moreover, uneven fragments were observed, which indicated the damages induced on bacterial cell membranes. Moreover, the treated S. aureus cell surfaces were uneven, the size of cells were reduced, and the cells seemed to be damaged. Through the SEM analysis, the present study confirmed that the MDR S. aureus membrane surfaces were damaged, upon the treatment with arborine and skimmianine compounds isolated from G. pentaphylla.
Figure 4.
The effect of arborine and skimmianine on the morphology of different MDR Staphylococcus aureus strains observed under scanning electron microscopy. (A) Control (without any treatment), (B) arborine-treated, and (C) skimmianine-treated S. aureus cells (the numbers on the images represent the S. aureus strain numbers).
Discussion
Medicinal plants have been the major source for innumerable therapeutic agents, which are of great importance to the health of an individual as well as communities (30). G. pentaphylla ethyl acetate crude extract was separated into nine fractions by thin-layer chromatography. Only two fractions showed excellent antibacterial property. This fraction was composed of two compounds that were visualized in TLC under the UV chamber. This study clearly shows that the active fractions obtained from column chromatographic separation are found to be mixtures of compounds. The purity of the fractions was obtained by column chromatography. The column purified compounds were characterized by 1H NMR, C13 NMR, FT-IR, HPLC, and LC-MS. The compounds GP-1 and GP-2 exactly matched the structure of arborine and skimmianine compounds with the molecular formula C16H14N2O and C14H13NO4, respectively. The 13C NMR also supports the presence of the N-methyl and benzylic group. The characteristic functional groups found at 1602 cm−1 (C=O) and 1402 cm−1 (C–N) in the FT-IR spectra confirmed the arborine structure. Finally, the LC-MS also complies with the mass of arborine (MW 250), which is observed as an M+1 peak (251).
Similarly, Chakravarti et al. (31) have isolated the alkaloid arborine compound from the leaves of Glycosmis arborea, and its structure was further confirmed as 2-benzyl-1-methylquinazol-4-one based on UV, FT-IR, and IHNMR analysis. The present findings correlated with the observations of the arborine compound isolated from the stem of G. pentaphylla, which was already reported by Chakravarti et al. (32), Govindachari et al. (33), and Muthukrishnana et al. (34). Ghani (35) reported that the G. pentaphylla leaves contain alkaloids arborine, arbornine, skimmianine, glycorine, glycophymine, glycophymoline, glycosmicine, and glycomide.
The structure of skimmianine was confirmed by 1H NMR, 13C NMR, FT-IR, and LC-MS. In 13C NMR, three methoxy carbons were observed at δ 56.7, 58.8, and 61.5 ppm. The FT-IR values of about 1,616 cm−1 (C=N) 1,266, 1,056 cm−1 (C–O, ether), and 1,056, 1,192 cm−1 (C-O, Furan) confirmed the structure of skimmianine. The LC-MS also confirmed the molecular weight of skimmianine, which was observed as M+1 peak (MW 260). The findings were in good agreement with earlier reports of Sreelekha (36) who isolated the skimmianine from Zanthoxylum rhetsa. Desai et al. (37) have isolated arborinine, cycleanine, isochondrodendrine, and skimmianine from the leaves of G. pentaphylla. Similarly, Sinhababu and Takur (38) have reported alkaloids arborine, arborinine, skimmianine, glycorine, glycosmicine, and an amide that was isolated from the flower heads of G. pentaphylla. On the other hand, Chakravarty et al. (39) isolated a skimmianine compound from G. arborea. Another study was done by Mester (40) and Greger et al. (41) isolated well-known alkaloids, arborine, and skimmianine compounds from G. parviflora.
The isolated arborine and skimmianine compounds show significant antibacterial activity against multidrug-resistant S. aureus isolates. Similar findings were reported by Bowen et al. (42), Chakravarti et al. (31), and Chakravarty et al. (39) who carried out the antibacterial activity of arborine and skimmianine compounds against both Gram-positive bacteria (S. aureus and MRSA) and Gram-negative bacteria (E. coli and S. typhimurium), which were inactive against S. aureus. Previously, Jeyachandran et al. (43) have reported that the plumbagin bioactive compound was isolated from the root extract of Plumbago zeylanica, exhibiting more toxic potential against S. aureus. The in vitro time-kill assay is one of the most commonly used experimental models to assess the antibacterial activity, efficiently characterizing the rate, extent, and timing of bacterial killing and regrowth (44). The time-kill test finds out the differences in the rate and extent of antibacterial activity over time, and it can also provide growth kinetics information (45). The overall result of this assay revealed a stronger bactericidal effect in arborine and skimmianine compounds against S. aureus than in a laboratory medium. The evaluation of protein leakage of the arborine and skimmianine showed a significant strong effect on the tested MDR S. aureus strains. A similar kind of protein leakage when treated with oil compounds from medicinal plants supports the findings of the present study (46).
The SEM result of the present study shows that bacterial cell membranes were significantly affected by the activity of arborine and skimmianine compounds. It is indicated that arborine and skimmianine bioactive compounds disturb the bacterial membrane and death to cells. Similar findings were reported by Campos et al. (47) who identified that the S. aureus and E. coli bacterial membranes and cytoplasm were highly affected due to the passive diffusion of plant metabolites into the cells that caused consequent cell disruption. These changes in bacteria cells may be due to the lysis of membrane and transformation caused by the damage on the permeability and integrity of membrane from arborine and skimmianine compounds. Consequently, the changes can lead to loss of inner cell materials (48, 49). The results of SEM were in good agreement with other findings of Paul et al. (50) and Sharma et al. (51) reported that other antimicrobials treated cells. Shen et al. (29) have reported that S. aureus damaged cell membrane affects the cell permeability, and outflow of cellular components that lead to cell death which supported the present study.
Conclusion
The present study indicated that G. pentaphylla is a good source of arborine and skimmianine, which acts as potent antibacterial agents against MDR S. aureus clinical isolates. Besides, these compounds induced significant strong bactericidal effects on the MDR S. aureus such as intracellular molecular imbalance and cell membrane disturbances that caused cell death. Hence, this study recommends that the isolated compounds can be used as a template molecule for pharmaceutical drug design for the treatment of diseases caused by MDR S. aureus.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
NM and RS equally contributed to the experiments and wrote the manuscript. AM, MK, and DN designed the research work and carry out the corrections in the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the Department of Biotechnology, Periyar University, Salem, Tamil Nadu, India, for providing necessary laboratory facilities, Tamil Nadu State Council for Science and Technology for financial support under the S&T Project Scheme (TNSCST/S&T Projects/RJ/BS/2013-2014), and DST-FIST (SR/FIST/LSI-673/2016) for strengthening the instrumentation facility of the Biotechnology Department of Periyar University. We thank Dr. S. Gnanavel, Department of Chemistry, Government Engineering College, Salem, for his technical assistance in the isolation of compounds and Dr. G. Ramkumar, ICAR-Indian Institute of Horticultural Research, for his suggestions on the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00176/full#supplementary-material
References
- 1.Plata K, Rosato AE, Wegrzyn G. Staphylococcus aureus as an infectious agent: overview of biochemistry and molecular genetics of its pathogenicity. Acta Biochim Pol. (2009) 56:597–612. 10.18388/abp.2009_2491 [DOI] [PubMed] [Google Scholar]
- 2.Shittu AO, Lin J, Kolawole DO. Antimicrobial susceptibility patterns of Staphylococcus aureus and characterization of MRSA in Southwestern Nigeria. Wounds. (2006) 18:77–84. [Google Scholar]
- 3.Price LB, Stegger M, Hasman H, Aziz M, Larsen J. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. Am Soc Microbiol. (2012) 52:139–46. 10.1128/mBio.00305-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deleo FR, Kennedy AD, Chen L. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci USA. (2011) 108:18091–6. 10.1073/pnas.1111084108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Antimicrobial resistance: global report on surveillance 2014. (2014). Available online at: https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf;jsessionid=E8D8B96F2905D8AFBDCBDE65C4D0D825?sequence=1
- 6.Xie X, Wong WW, Tang Y. Improving simvastatin bioconversion in Escherichia coli by deletion of bioH. Metab Eng. (2007) 9:379–86. 10.1016/j.ymben.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 7.Alviano DS, Alviano CS. Plant extracts: search for new alternatives to treat microbial diseases. Curr Pharm Biotechnol. (2009) 10:106–21. 10.2174/138920109787048607 [DOI] [PubMed] [Google Scholar]
- 8.Junio HA, Sy-Cordero AA, Ettefagh KA, Burns JT, Micko KT, Graf TN, et al. (2011). Synergy-directed fractionation of botanical medicines: a case study with goldenseal (Hydrastis canadensis). Nat Prod. 74:1621–9. 10.1021/np200336g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J, Choi J, Mun S, Kang O, Preeti B, Dong-Won S, et al. Antimicrobial activity and synergism of Sami-Hyanglyun-Hwan with ciprofloxacin against methicillin-resistant Staphylococcus aureus. Asian Pac J Trop Med. (2008) 8:538–42. 10.1016/j.apjtm.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 10.Santos PRV, Oliveira ACX, Tomassini TCB. Controle microbiógico de produtos fitoterápicos. Rev Farm Bioquím. (1995) 31:35–8. [Google Scholar]
- 11.Suganya A, Murugan K, Kovendan K, Mahesh Kumar P, Hwang JS. Green synthesis of silver nanoparticles using Murraya koenigii leaf extract against Anopheles stephensi and Aedes aegypti. Parasitol Res. (2013) 112:1385–97. 10.1007/s00436-012-3269-z [DOI] [PubMed] [Google Scholar]
- 12.Kuzel S, Vydra J, Triska J, Vrchotova N, Hruby Mand Cigler P. Elicitation of pharmacologically active substances in an intact medical plant. J Agri Food Chem. (2009) 57:7907–11. 10.1021/jf9011246 [DOI] [PubMed] [Google Scholar]
- 13.Taware AS, Mukadam DS, Chavan AM, Taware SD. Comparative studies of in vitro and in vivo grown plants and callus of Stevia rebaudiana (Bertoni). Int J Integr Biol. (2010) 9:10–5. [Google Scholar]
- 14.Dakah A, Suleiman M, Zaid S. Effect of some Environmental stress in tissue culture media on in vitro propagation and antioxidant activity of medicinal plant Ziziphora canescens Benth. Adv Nat Appl Sci. (2014) 8:224–32. [Google Scholar]
- 15.Murugan N, Natarajan D. Phytochemical, antioxidant and antibacterial activities of Glycosmis pentaphylla (Rutaceae) leaf extracts against selected multi-drug resistant bacteria. J Chem Pharm Res. (2016) 8:737–44. [Google Scholar]
- 16.Murugan N. Antibacterial activity of Glycosmis Pentaphylla plant against clinically isolated multi drug resistant Staphylococcus aureus and their in silico analysis (Ph.D Thesis). Periyar University, Salem, Tamil Nadu; (2016). p. 97–110. [Google Scholar]
- 17.Srinivasan R. Bioactivity guided isolation and structural elucidation of antimicrobial, antioxidant and larvicidal compounds from Elaeagnus indica and Memecylon edule and their molecular docking studies (Ph.D Thesis). Periyar University, Salem, Tamil Nadu; (2014). p. 20–43. [Google Scholar]
- 18.Srinivasan R, Natarajan D, Shivakumar MS, Vinuchakkaravarthy T, Velmurugan D. Bioassay-guided isolation of mosquito larvicidal compound from acetone leaf extract of Elaeagnus indica Servett Bull and its in-silico study. Ind Crops Prod. (2015) 76:394–401. 10.1016/j.indcrop.2015.07.032 [DOI] [Google Scholar]
- 19.Srinivasan R, Natarajan D, Shivakumar MS. Spectral characterization and antibacterial activity of an isolated compound from Memecylon edule leaves, J. Photochem Photobiol B Biol. (2017) 168:20–4. 10.1016/j.jphotobiol.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan R, Natarajan D, Shivakumar MS, Nagamurugan N. Isolation of fisetin from Elaeagnus indica Servett. Bull. (Elaeagnaceae) with antioxidant and antiproliferative activity. Free Radic Antioxid. (2016) 6:145–50. 10.5530/fra.2016.2.3 [DOI] [Google Scholar]
- 21.Natarajan D, Srinivasan R, Shivakumar MS. Gas chromatography mass spectroscopy chromatogram and antimicrobial activity of leaf extracts of Blepharis maderaspatensis and Maesa indica. J Herbs Spices Med Plants. (2015) 21:267–82. 10.1080/10496475.2014.960637 [DOI] [Google Scholar]
- 22.Parsons HM, Ludwig C, Gunther UL, Viant MR. Improved classification accuracy in 1- and 2-dimensional NMR metabolomics data using the variance stabilising generalised logarithm transformation. BMC Bioinform. (2007) 8:234. 10.1186/1471-2105-8-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawry MA, Soczewinski E, Dzido TH. Separation of coumarins from Archangelica officinalis in high-performance liquid chromatography and thin-layer chromatography system. J. Chromatogr A. (2000) 366:75–81. 10.1016/S0021-9673(00)00321-6 [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan R, Natarajan D, Shivakumar MS. Antimicrobial and GC-MS analysis of Memecylon edule leaf extracts. Int J Curr Pharma. Rev Res. (2014) 5:1–13. 10.5530/fra.2015.1.6 [DOI] [Google Scholar]
- 25.Natarajan D, Srinivasan R, Shivakumar MS. Phyllanthus wightianus Müll. Arg - a potential source for natural antimicrobial agents. Biomed Res Int. (2014) 2014:135082. 10.1155/2014/135082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balakumaran MD, Ramachandran R, Balashanmugam P, Mukeshkumar DJ, Klaichelvan PT. Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microb Res. (2016) 182:8–20. 10.1016/j.micres.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 27.García P, Martínez B, Rodríguez L, Rodríguez A. Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. Int. Food Microbiol. (2010) 141:151–5. 10.1016/j.ijfoodmicro.2010.04.029 [DOI] [PubMed] [Google Scholar]
- 28.Dos Santos AO, Ueda-Nakamura T, Dias BP, Veiga VF, Pinto AC, Nakamura CV. Antimicrobial activity of Brazilian copaiba oils obtained from different species of the Copaifera genus. Mem Inst Oswaldo Cruz. (2008) 103:277–81. 10.1590/S0074-02762008005000015 [DOI] [PubMed] [Google Scholar]
- 29.Shen SX, Zhang TH, Yuan Y, Lin SY, Xu JY, Ye HQ. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control. (2015) 47:196–202. 10.1016/j.foodcont.2014.07.003 [DOI] [Google Scholar]
- 30.Ganesan S. Traditional oral care medicinal plant survey of Tamilnadu. Nat Prod. Radiance. (2008). p. 7:166–72. [Google Scholar]
- 31.Chakravarti D, Chakravarti RN, Cohen LA, Dasgupta B, Datta S, Miller HK. Alkaloids of Glycosmis arborea-II. Structure of arborine. Tetrahedron. (1961) 16:224–50. 10.1016/0040-4020(61)80074-4 [DOI] [Google Scholar]
- 32.Chakravarti D, Chakravarti RN, Chakravari SC. Alkaloids of Glycosmis arborea. Part I Isolation of arborine and arborinine: the structure of arborine. J Chem Soc. (1953) 3337 10.1039/jr9530003337 [DOI] [Google Scholar]
- 33.Govindachari TR, Jadhav SJ, Joshi BS, Kamat VN, Mohamed PA, Parthasarathy PC, et al. Chemical investigation of some Indian Plants. Indian J Chem. (1969) 7:308–9. [Google Scholar]
- 34.Muthukrishnana J, Seifert K, Homannc KH, Lorenz MW. Inhibition of juvenile hormone biosynthesis in Gryllus bimaculatus by Glycosmis pentaphylla leaf compounds. Phytochemistry. (1999) 50:249–54. 10.1016/S0031-9422(98)00537-8 [DOI] [Google Scholar]
- 35.Ghani A. Medicinal plants of Bangladesh. Asiatic Soc. (2003) 430:502–4. [Google Scholar]
- 36.Sreelekha M (2012). Studies on secondary plant metabolites and their biological properties. (Ph.D thesis). Department of Chemistry, University of Calicut, Tirur, Kerala. [Google Scholar]
- 37.Desai PD, Dutta MD, Ganguly AI, Govindachary TR, Joshi BS, Kamat VN, et al. Chemical investigation of some Indian plants: Part III. Indian J Chem. (1967) 5:523–4. [Google Scholar]
- 38.Sinhababu A, Thakur S. Constituents of flower of Glycosmis pentaphylla (Retz) Correa. Asian J Chem. (1995) 7:221–2. [Google Scholar]
- 39.Chakravarty AK, Sarkar T, Masuda K, Shiojima K. Carbazole alkaloids from root of Glycosmis arborea. Phytochemisty. (1999) 50:1263–6. 10.1016/S0031-9422(98)00666-9 [DOI] [Google Scholar]
- 40.Mester I. Structural diversity and distribution of alkaloids in the Rutales. In: Waterman PG, Grundon ML, editors. Chemistry and Chemical Taxonomy of the Rutales. New York, NY; London: Academic Press; (1983). p. 31–96. [Google Scholar]
- 41.Greger H, Zechner G, Hofer O, Hadacek F, Wurz G. Sulphur-containingamides from Glycosmis species with different antifungal activity. Phytochemistry. (1993) 34:175–9. 10.1016/S0031-9422(00)90802-1 [DOI] [Google Scholar]
- 42.Bowen IH, Perera CKPW, Lewis JR. Alkaloids of the leaves of Glycosmis bilocularis. Phytochemistry. (1978) 17:2125–7. 10.1016/S0031-9422(00)89294-8 [DOI] [Google Scholar]
- 43.Jeyachandran R, Mahesh A, Cindrella L, Sudhakar S, Pazhanichamy K. Antibacterial activity of plumbagin and root extracts of Plumbago zeylanica L. Acta Biol Cracoviens Ser Bot. (2009) 51:17–22. [Google Scholar]
- 44.Cheahe D, Zhang Y, Gao D, Zhang H. Antibacterial and anti-inflammatory activities of extract and fractions from Pyrrosia petiolosa (Christ et Bar.) Ching. J Ethnopharmacol. (2015) 155:1300–05. 10.1016/j.jep.2014.07.029 [DOI] [PubMed] [Google Scholar]
- 45.Lewis S, Tarrier N, Haddock G, Lewis S, Tarrier N, Haddock G. Randomised controlled trial of cognitive-behavioural therapy in early schizophrenia: acute-phase outcomes. Br J Psychiatry. (2002) 181:s91–7. 10.1192/bjp.181.43.s91 [DOI] [PubMed] [Google Scholar]
- 46.Lattaoui N, Tantaoui-Elaraki A. Individual and combined antibacterial activity of the main components of three thyme essential oils. Riv Ital EPPOS. (1994) 13:13–9. [Google Scholar]
- 47.Campos FM, Couto JA, Figueiredo AR, Tóth IV, Rangel AOSS, Hogg TA. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int J Food Microbiol. (2009) 135:144–51. 10.1016/j.ijfoodmicro.2009.07.031 [DOI] [PubMed] [Google Scholar]
- 48.Bajpai VK, Al-Reza SM, Choi UK, Lee JH, Kang SC. Chemical composition, antibacterial and antioxidant activities of leaf essential oil and extracts of Metasequioa glyptostroboides Miki ex Hu. Food Chem Toxicol. (2009) 47:1876–83. 10.1016/j.fct.2009.04.043 [DOI] [PubMed] [Google Scholar]
- 49.Shin SY, Bajpai VK, Kim HR, Kang SC. Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int J Food Microbiol. (2007) 113:233–6. 10.1016/j.ijfoodmicro.2006.05.020 [DOI] [PubMed] [Google Scholar]
- 50.Paul S, Dubey RC, Maheswari DK, Kang S. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control. (2011) 22:725–31. 10.1016/j.foodcont.2010.11.003 [DOI] [Google Scholar]
- 51.Sharma A, Bajpai VK, Baek K. Determination of antibacterial mode of action of Allium sativum essential oil against foodborne pathogens using membrane permeability and surface characteristic parameters. J Food Saf. (2013) 33:197–208. 10.1111/jfs.12040 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.