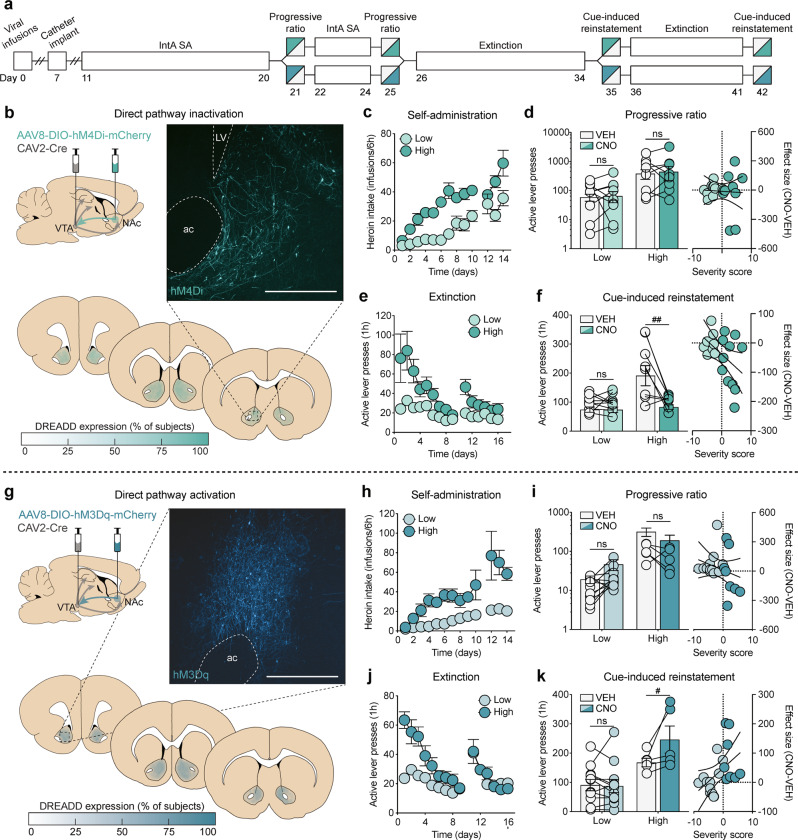
Fig. 4.
Transient modulation of dMSNs bidirectionally alters reinstatement but not motivation in high-risk rats. a Timeline for chemogenetic modulation of motivation under a PR schedule of reinforcement and cue-induced reinstatement of drug-seeking. b Viral strategy, representative histology, and quantification of expression for dMSN inactivation. c–f Self-administration, progressive ratio, extinction, and reinstatement data for dMSN-hM4Di rats (low: n = 9; high: n = 9). d dMSN inactivation has no effect on motivation. Left: High-risk rats have greater responding for heroin than low-risk rats, which is unaffected by dMSN inactivation; right: dMSN-hM4Di has no effect on motivation, regardless of addiction severity. f dMSN inactivation selectively reduces reinstatement in high-risk rats. Left: High-risk rats have greater responding for heroin cues than low-risk rats, which is selectively attenuated by CNO; right: the effect size of dMSN-hM4Di significantly correlates with addiction severity. g Viral strategy, representative histology, and quantification of expression for dMSN activation. h–k Self-administration, progressive ratio, extinction, and reinstatement data for dMSN-hM3Dq rats (low: n = 11; high: n = 6). i dMSN activation has no effect on motivation. Left: High-risk rats have greater responding for heroin than low-risk rats, which is unaffected by dMSN activation; right: dMSN-hM3Dq has no effect on motivation, regardless of addiction severity. (k) dMSN activation selectively enhances reinstatement in high-risk rats. Left: High-risk rats have greater responding for heroin cues than low-risk rats, which is further enhanced by CNO; right: the effect size of dMSN-hM3Dq significantly correlates with addiction severity. Scale bar = 500 μm; ac , anterior commissure; LV, lateral ventricle; #p < 0.05 (VEH vs CNO), ##p < 0.01 (VEH vs CNO).