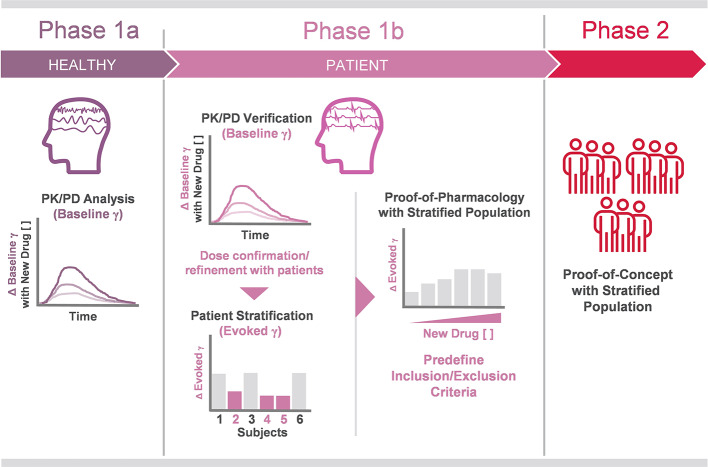
Figure 1.
Proposed workflow of gamma oscillation analysis in future clinical trials. Phase 1a PK/PD analysis of investigational drugs targeting E/I imbalance. In the proposed trial design, baseline gamma alterations are tracked against investigational drugs concentrations in plasma, receptor occupancy, etc., in healthy volunteers. These results are confirmed in the patient population prior to stage-up. Phase 1b also includes patient population stratification, achieved in part by identification of blunted evoked gamma in ASSR testing. Phase 2 trial design is informed on dosing and time course insights gained in Phase 1a/b PK/PD testing, while candidate patient populations are defined (Phase 1b) before therapeutic response is evaluated using evoked gamma ASSR and other clinical endpoints in Phase 2.