Figure 2. B2 cells generate C3a/C5a from endogenously produced complement and autocrine C3ar1/C5ar1 signaling in B2 cells is needed for Ab production.
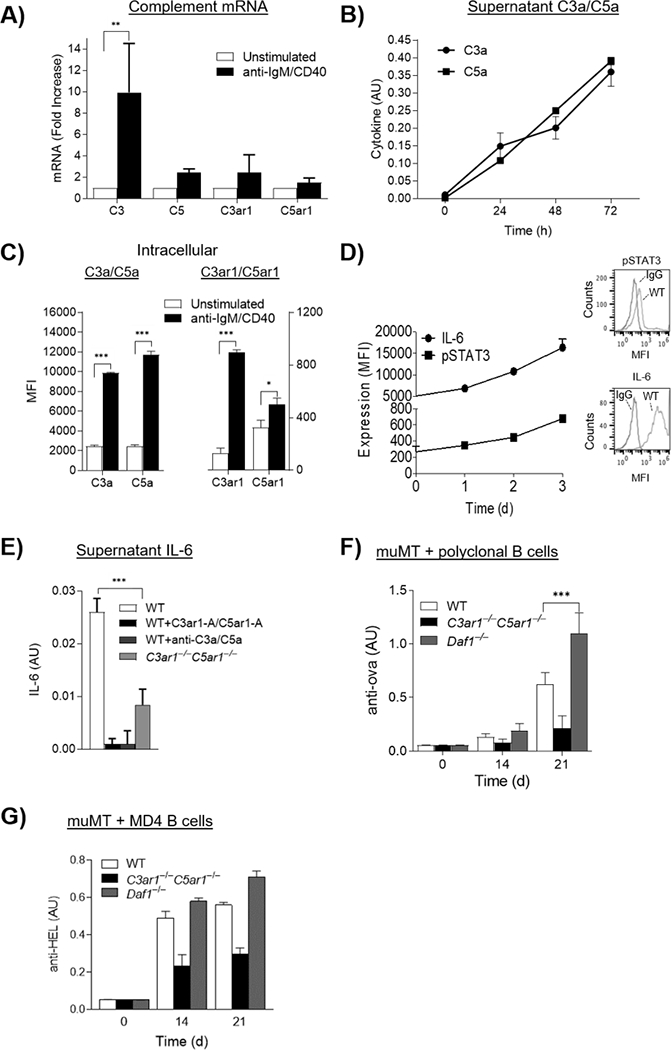
A) WT splenic B2 cells were incubated with anti-IgM F(ab’)2/anti-CD40 (3 μg/ml each) for 24 h at 37°C after which C3/C3ar1/C5/C5ar1 mRNA expression were quantified by qPCR. (n=3) p<05 B) Culture supernatants of the cells in A) were assayed for C3a and C5a by ELISA over time. N=3, p<.05 C) Unstimulated and anti-IgM (Fab’)2 stimulated WT B2 cells were assayed for intracellular C3a, C3ar1, C5a, C5ar1 by flow cytometry of permeabilized cells. n=3, p<.05. D) WT B2 cells were stimulated as in A) and intracellular IL-6 and p-STAT3 were assayed by FACS of permeabilized cells. n=3, p<.05 E) Anti-IgM (Fab’)2/CD40 stimulated WT B2 cells were incubated with media alone, C3ar1-A/C5ar1-A (30 ng/ml each), or anti-C3a/C5a mAbs (3 ug/ml each) and identically stimulated C3ar1−/−C5ar1−/− B2 cells were incubated with media alone after which supernatants were assayed for IL-6 by ELISA. AU =Absorbance units, n=3, p,.05. F) WT, C3ar1−/−C5ar1−/−, or Daf1−/− B2 cells (3×106) were adoptively transferred into muMT recipients (n=5 respectively). Recipients were then immunized with ova/CFA and assayed at 0, 14, and 21 d for anti-ova Ab by ELISA. The anti-ova Ab is total Ig. G) MD4 B2 cells on the WT, C3ar1−/−C5ar1−/−, or Daf1−/− backgrounds (n=5 each) were transferred to another set of muMT recipients after which the animals were immunized with HEL/CFA. Anti-HEL IgM Ab levels were assayed at 0, 14, and 21 d by ELISA.
