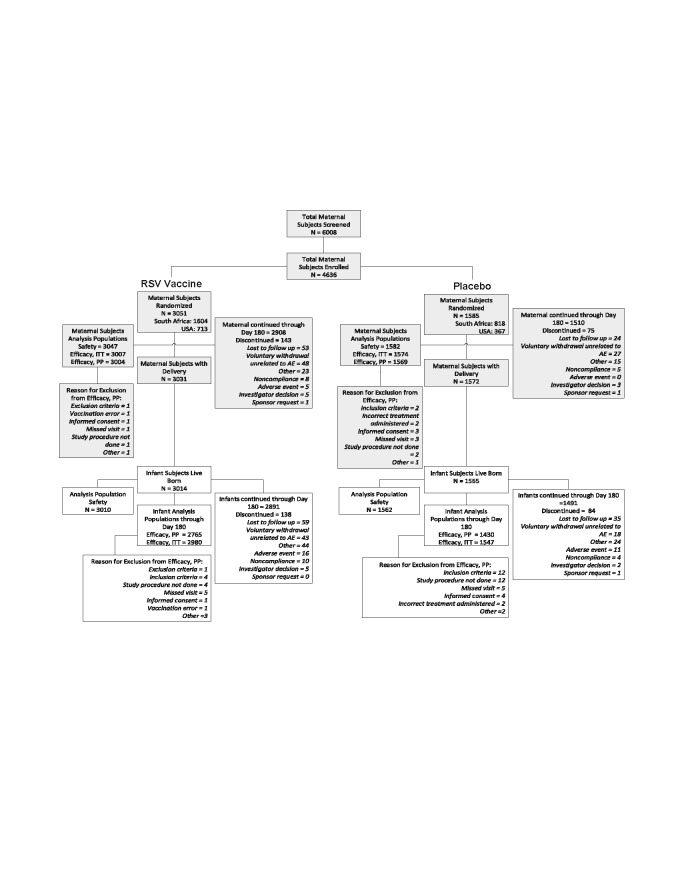
Figure 1.
Consort diagram on screening, enrolment and disposition of subjects.
The maternal safety population included all maternal subjects who received any test article. Infant safety population was all infants born live to maternal subjects who received any test article.
The per-protocol efficacy population for maternal subjects was all maternal subjects who received the test article and regimen to which they were randomized and had at least one post-treatment encounter documented during which active and/or passive surveillance activities for RSV-suspect illness could occur, and had no major protocol deviations affecting the primary efficacy outcomes as determined and documented by Novavax prior to database lock and unblinding.
The per-protocol efficacy population for infant subjects included all infant subjects who: a) were ≥ 37 weeks gestational age at birth, b) were born to maternal subjects who received a study injection as randomized and ≥ 2 weeks prior to delivery, c) had not received prophylactic treatment with palivizumab between birth and Day 180 after delivery, d) had at least one post-partum contact during which active and/or passive surveillance activities for RSV-suspect illness could occur, and e) had no major protocol deviations affecting the primary efficacy outcomes as determined and documented by Novavax prior to database lock and unblinding.
The intent-to-treat efficacy population included all maternal subjects and their infants in the Safety Population for whom at least one post-treatment and post-partum, respectively, efficacy measurement was available for both the mother and the infant as evidenced by collection of surveillance observations.