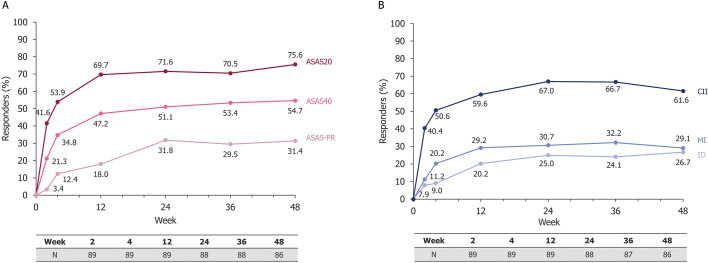
Figure 3.
(A) ASAS20, ASAS40 and ASAS partial remission responder rates up to Week 48 in axSpA patients receiving CZP 200 mg Q2W and (B) ASDAS, CII, MI, and ID responder rates up to Week 48 in axSpA patients receiving CZP 200 mg Q2W. Safety Set (N=89). Observed data are shown; total number of patients assessed at each timepoint is shown. ASAS, Assessment of SpondyloArthritis international Society; ASAS20/40, ASAS 20%/40% response; axSpA, axial spondyloarthritis; CII, clinical important improvement; CZP, certolizumab pegol; ID, inactive disease; MI, major improvement; PR, partial remission; Q2W, every 2 weeks.