FIGURE 2.
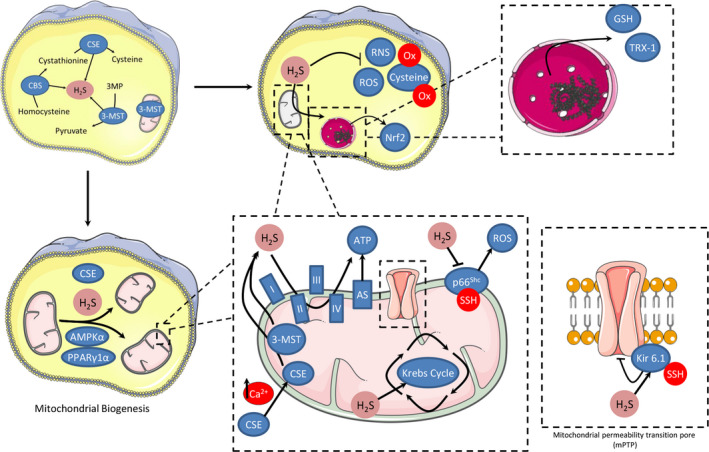
Proposed sources and targets for mitochondrial H2S generation involved in cardioprotection. H2S can be generated from 3‐mercaptopyruvate sulphurtransferase (3‐MST), that has been found in both cytosol and mitochondria and from the translocation of cystathionine γ‐lyase (CSE) from the cytosol to mitochondria after prolonged elevation of Ca2+ levels. H2S induces cardioprotection by preservation of mitochondrial function: H2S can inhibit ROS and RNS formation preventing irreversible cysteine overoxidation and preserving protein functions. H2S activates the master‐regulator of antioxidant responses Nrf2, increases glutathione (GSH) synthesis and up‐regulates the expression of thioredoxin. H2S may act as an endogenous antioxidant mediator by inhibition of p66Shc‐mediated mitochondrial ROS production. Another possible mechanism of action for H2S is based on its ability to modulate cellular respiration during reperfusion. Under physiological H2S concentrations, cytochrome c oxidase remains functional, whereas sulphide oxidation likely contributes to mitochondrial ATP production. Additionally, H2S regulates mitochondrial biogenesis by activation of AMP‐activated protein kinase and peroxisome proliferator‐activated receptor γ coactivator 1α. H2S modulates cellular signalling by sulfhydration, and among the proteins confirmed to undergo sulfhydration upon exposure to H2S, several are involved in cardioprotection including the pore forming subunit of ATP‐sensitive potassium channels (Kir 6.1)
