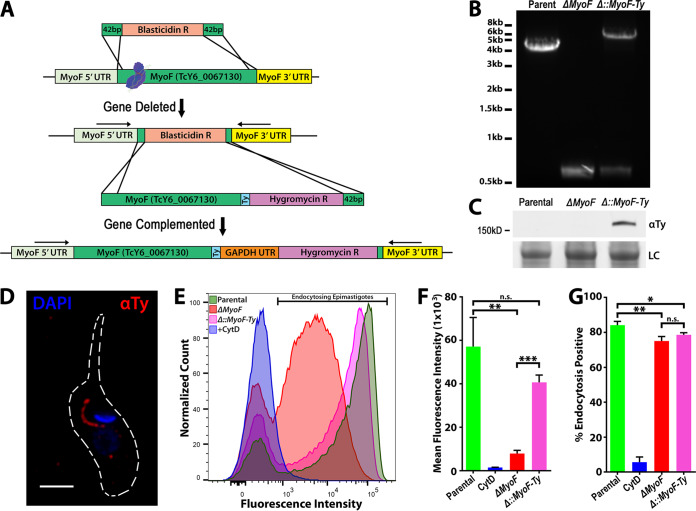
FIG 4.
MyoF deletion mutants exhibit a reduction in the rate of SPC endocytosis. (A) Scheme for CRISPR/Cas9 gene replacement and complementation strategy used to generate ΔMyoF deletion and complemented (Δ::MyoF-Ty) mutants. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) PCR amplification of the genomic locus showing replacement of both parental loci (high-molecular-weight [MW] band) with the blasticidin resistance gene (low-MW band) in a subclone of the ΔMyoF mutant. PCR of a nonsubcloned population of complemented parasites shows insertion of the MyoF-Ty-Hygro repair template into the MyoF locus. (C) Anti-Ty immunoblot of lysates from parental, ΔMyoF, and (Δ::MyoF-Ty) epimastigotes showing expression of MyoF-Ty in the complemented parasites. (D) Immunofluorescence assays of a (Δ::MyoF-Ty) epimastigote showing expression and proper SPC localization of MyoF-Ty. (E) Flow cytometry analysis of epimastigotes fed fluorescent BSA showing a reduced rate of feeding in ΔMyoF mutants, a phenotype that is partially rescued in the complemented (Δ::MyoF-Ty) mutants. (F) Quantification of the feeding rate results represented by reduced mean fluorescence of endocytosing epimastigotes. A dramatic reduction in the feeding rate was seen in the ΔMyoF mutants, which was partially rescued by complementation (Δ::MyoF-Ty). (G) A minor but statistically significant percentage of the total number of ΔMyoF and (Δ::MyoF-Ty) epimastigotes showed reduced levels of endocytosed fluorescent BSA during the assay.