Abstract
Objective
To evaluate the effects of a computerised decision support tool for comprehensive drug review in elderly people with polypharmacy.
Design
Pragmatic, multicentre, cluster randomised controlled trial.
Setting
359 general practices in Austria, Germany, Italy, and the United Kingdom.
Participants
3904 adults aged 75 years and older using eight or more drugs on a regular basis, recruited by their general practitioner.
Intervention
A newly developed electronic decision support tool comprising a comprehensive drug review to support general practitioners in deprescribing potentially inappropriate and non-evidence based drugs. Doctors were randomly allocated to either the electronic decision support tool or to provide treatment as usual.
Main outcome measures
The primary outcome was the composite of unplanned hospital admission or death by 24 months. The key secondary outcome was reduction in the number of drugs.
Results
3904 adults were enrolled between January and October 2015. 181 practices and 1953 participants were assigned to electronic decision support (intervention group) and 178 practices and 1951 participants to treatment as usual (control group). The primary outcome (composite of unplanned hospital admission or death by 24 months) occurred in 871 (44.6%) participants in the intervention group and 944 (48.4%) in the control group. In an intention-to-treat analysis the odds ratio of the composite outcome was 0.88 (95% confidence interval 0.73 to 1.07; P=0.19, 997 of 1953 v 1055 of 1951). In an analysis restricted to participants attending practice according to protocol, a difference was found favouring the intervention (odds ratio 0.82, 95% confidence interval 0.68 to 0.98; 774 of 1682 v 873 of 1712, P=0.03). By 24 months the number of prescribed drugs had decreased in the intervention group compared with control group (uncontrolled mean change −0.42 v 0.06: adjusted mean difference −0.45, 95% confidence interval −0.63 to −0.26; P<0.001).
Conclusions
In intention-to-treat analysis, a computerised decision support tool for comprehensive drug review of elderly people with polypharmacy showed no conclusive effects on the composite of unplanned hospital admission or death by 24 months. Nonetheless, a reduction in drugs was achieved without detriment to patient outcomes.
Trial registration
Current Controlled Trials ISRCTN10137559.
Introduction
Polypharmacy is common in older adults, particularly those aged 65 or older, and, despite not always being avoidable, often includes inappropriate drugs and carries a risk of unfavourable health outcomes.1 Clinicians therefore have to balance the risks and benefits of prescribing multiple drugs, taking into account drug-drug and disease-drug interactions.2
Given the high number of older people with polypharmacy in primary care 1 3 and the limited time that general practitioners have to carry out regular drug reviews, alternative approaches such as computerised decision support systems are needed to optimise drug treatment and to facilitate deprescribing.4 Although such systems have been developed, they usually target specific drugs or groups of drugs and in general have not been found to be effective.5 Previous research on the appropriate use of drugs in people with polypharmacy consists of a range of interventions in several settings using diverse study designs and outcomes.4 5 Few interventions have provided electronic decision support to doctors in their own practice.4 Although some individual studies have shown positive effects in drug appropriateness, in general the interventions had little or no effect on hospital admissions4 or on all cause mortality.5 We developed an easy to use computerised decision support system designed specifically to deal with potentially inappropriate polypharmacy. This system, called PRIMA-eDS (polypharmacy in chronic diseases: reduction of inappropriate medication and adverse drug events in older populations by electronic decision support), automatically generates a comprehensive drug review based on individual patient data and current best evidence6 to support doctors in deprescribing.
In this multicentre cluster randomised controlled trial we tested whether use of the electronic decision support tool led to a reduction in inappropriate polypharmacy and thus improvements in patient relevant endpoints. Our principal hypothesis was that deprescribing based on the electronic decision support tool would reduce the composite endpoint of unplanned hospital admission or death in patients aged 75 or older.
Methods
Study design and participants
This pragmatic cluster randomised controlled trial was carried out in four countries. General practitioners were recruited in five study centres: Manchester, United Kingdom; Bolzano, Italy; Salzburg, Austria; and Rostock and Witten, Germany. The doctors were recruited between May 2013 and September 2015. Participating doctors in turn recruited patients aged 75 or older who were using eight or more drugs on a regular basis (including those prescribed by any doctor, and non-prescription drugs). We decided to use the cut-off point of at least eight drugs based on a previous study.7 8 Patients were excluded if they had a life expectancy of less than 12 months, were unable to give informed consent, or were receiving chemotherapy or radiation for systemic malignant disease.
Doctors in prespecified regions around each study centre were informed about the trial and invited to participate. After they had agreed to participate and provided written informed consent, we asked them to identify all potentially eligible patients in their practice and to check them for inclusion and exclusion criteria. Group practices were limited to one participating doctor only. The doctors were then asked to recruit 11 eligible patients at their discretion to participate in the trial. Recruiting procedures varied slightly between centres according to local conditions.
Overall, 3904 patients were enrolled between 7 January and 5 October 2015. A more detailed description of the PRIMA-eDS trial protocol is available elsewhere.9 Further details of the trial’s electronic decision support tool and the randomised controlled trial have been presented elsewhere,10 11 and baseline data have been published previously.12
Randomisation and masking
After patient recruitment and baseline data collection had been completed, we randomised the doctors to either the electronic decision support tool (intervention group) or treatment as usual (control group). Randomisation was at doctor level to avoid contamination should a doctor use the decision support tool for some patients but not for others. A trial statistician masked to allocation performed computerised block randomisation, stratified by study centre to ensure balanced groups. Owing to the nature of the intervention, doctors, participants, and study assistants could not be blinded, but statisticians undertaking analyses were masked.
Procedures
The trial’s observation period was two years. Follow-up patient consultations and data collection took place at 8, 16, and 24 months. At each time point the doctor or an authorised staff member collected patient data and entered these into an electronic case report form (see appendix fig 3a). At some UK practices, a regional Clinical Research Network research nurse working with the practice assisted in data collection. During a routine visit to the practice, the participant completed on paper the SF-12v2 health survey, a shortened version of the second version of the SF-36.
The intervention consisted of a computerised decision support tool providing a comprehensive drug review (see appendix figs 1a and 2a) generated from patient data recorded in the electronic case report form. The intervention is a novel and comprehensive approach to reducing potentially inappropriate and non-evidence based polypharmacy. It is not limited to any specific drug or drug classes but rather uses an all inclusive approach to a patient’s drugs. It provides a check of the indications for current drugs based on recorded diagnoses; a summary of measurement results with alerts; recommendations about amending current drugs according to best available evidence; advice on dosage adjustment in renal malfunction; alerts for potentially harmful drug-drug interactions; warnings for possible contraindications; dose warnings; and a table listing each current drug and the associated degree of risk for nine common adverse drug reactions (see table 1). Although the decision support tool was intended to encourage deprescribing of potentially inappropriate drugs, the final decisions about treatment remained at the discretion of the doctor and patient in a shared decision making process. The electronic decision support tool was web based and could also be saved as a PDF and printed.
Table 1.
Components of the computerised decision support tool for comprehensive drug review in people with polypharmacy for chronic diseases
| Components | Data sources |
|---|---|
| Check of indications for current drugs | Evidence-Based Medicine Guidelines and evidence summary collection13 |
| Measurement results (laboratory, anthropometric) with alerts | Evidence-Based Medicine Guidelines13 and consensus of EBMeDS clinical editorial team14 |
| Recommendations about amending current drugs based on best available evidence | EBMeDS evidence based rules and reminders.15 Systematic reviews on drugs commonly prescribed to older people6
16-21
EU(7)-PIM list22 |
| Dosage adjustment in renal malfunction | RENBASE database23 |
| Potentially harmful drug-drug interactions | INXBASE database24 |
| Contraindications | Pharmacological literature and summary of medicinal product characteristics by European Medicines Agency25 |
| Dose warnings | Pharmacological literature and product summaries approved by regulatory authorities |
| Possible adverse drug reactions (risk of bleeding, renal toxicity, risk of seizures, anticholinergic effects, constipation, orthostatism, QT prolongation, sedation, serotonergic effect) | RISKBASE database26 |
EbMeDS=evidence based medicine electronic decision support; EU(7)-PIM=European Union (7)-potentially inappropriate medications.
The baseline data collected on each participant consisted of age, sex, body mass index, blood pressure, all drugs (prescribed and over the counter) with an ATC (anatomic therapeutic chemical) code,27 all diagnoses (using the international classification of diseases, 10th revision (ICD-10) codes28), smoking status, current symptoms (within past month), medical procedures, frailty (clinical frailty scale29), number of falls (during past three months), and laboratory values (creatinine, blood glucose, alanine aminotransferase, glycated haemoglobin (HbA1c), platelet count, international normalised ratio, cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, potassium, and sodium) when available. We also recorded health related quality of life (SF-12v2: physical and mental component scores30) and educational level (international standard classification of education 1997 (ISCED-97)31). The participating doctors were advised to collect up to date data at a routine appointment. All these data, including primary and secondary outcomes, were recorded by the doctors or their staff in the electronic case report form and stored centrally on a server designated to this study.
After randomisation, doctors in the intervention group were given access to the computerised decision support tool. They received training and instructions on use, interpretation of the output, the evidence base underpinning the output, and the principles of shared decision making. Training was provided face to face, by webinar and by video tutorials through the PRIMA-eDS webpage. A telephone hotline was available to doctors in case of questions. Doctors in the intervention group were instructed to use the electronic decision support tool directly after randomisation, and at all three follow-up consultations. Furthermore, they were free to use the electronic decision support tool at any other time point.
Doctors in the control group treated participants according to their normal practice and only completed electronic case report forms at the scheduled follow-up visits. They were free to change any drug if they thought it appropriate, but they did not have access to the electronic decision support tool to guide their decision making.
Doctors in both groups were instructed to collect the data during routine appointments. They were not specifically asked to make extra appointments whenever possible for the study, but were free to do so if they wished.
Outcomes
The primary outcome was a composite of unplanned hospital admission or death from any cause during the two year observation period. We combined these measures into a composite endpoint because they can be competing outcomes. The key secondary outcome was the number of drugs prescribed at final follow-up, controlling for baseline, in line with the key objective of the intervention to reduce inappropriate prescribing. Other secondary outcomes were unplanned hospital admission and all cause mortality (as single endpoints), self-reported falls, recorded fractures, adverse drug reactions (symptoms), and quality of life. We further examined the number and types of recommendations given by the decision support tool to improve drug appropriateness.
At each follow-up visit the practice staff collected data pertaining to each outcome and entered these into the electronic case report form, with the exception of the SF-12, which was collected on a paper form at eight and 24 months. Practice staff recorded ongoing data on patients who dropped out or died.
Statistical analysis
The sample size was based on binary analysis to test superiority of the primary composite endpoint on unplanned hospital admission or death within two years. We powered the study to detect a relative risk reduction of 20% in the decision support group at 80% power and an α level of 5%, assuming an intracluster correlation coefficient of 0.01 (based on an earlier cluster trial32) and a 10% attrition rate in both patients and practices.9 On this basis we required 3542 patients across 322 general practices with a mean of 11 participants in each practice. About 10% excess recruitment over this was achieved in the actual trial.
An independent statistician blind to trial group allocation analysed the data following a prespecified statistical analysis plan. Mixed effect multilevel models were applied, with participants nested within general practices, grouped within study centres. We treated the practices as a random effect and the five centres as fixed effects.
We applied linear models for continuous outcomes, logistic for binary outcomes, Poisson or negative binomial (when the Poisson was not a good fit) for counts, and Cox regression for time-to-event outcomes. Baseline outcome values were included as covariates in the relevant model when available (number of falls, number of drugs, SF-12 physical and mental component scores) along with four prespecified covariates, judged prognostic of outcomes: participant sex, age, number of drugs prescribed at baseline, and number of medical diagnoses, with the last three treated as continuous variables. These and all other collected variables were well balanced between trial arms (see table 2).
All models included a fixed factor for study centre in line with the randomisation scheme and to control for any within country imbalance in patient numbers between trial arms. Baseline data indicated high heterogeneity in patient populations between countries, so to reduce risk of bias from factors such as Simpson’s paradox, we also adjusted for the interaction between treatment group and study centre. We then used a post-estimation contrast to estimate and test the mean effect between trial arms.
Baseline data were missing only on one variable—number of diagnoses—and for only six participants (0.15%). Before further analysis we imputed these missing values using simple linear regression based on age, sex, number of drugs, and study centre.33 In line with the trial protocol,9 we analysed missing 24 month follow-up data on the primary outcome as reaching the endpoint, with sensitivity analysis performed using multiple imputation with the chained equations procedure and 10 multiple imputation datasets, utilising the full set of variables, including the interaction term between group and study centre. For the key secondary outcome of number of drugs, where 24 month follow-up data was missing we used the last recorded value (at eight or 16 months) and controlled for number of consultations attended; we also conducted a multiple imputation sensitivity using imputed values at 24 months.
For other secondary outcomes, when 24 month follow-up data were missing we analysed the last recorded outcome score and, when appropriate (ie, for number and duration of hospital admission and number of falls), controlled for length of time in the trial as an exposure factor. We treated SF-12 scores as completely missing if they were not collected at 24 months.
When the data suggested that an outcome lacked adequate fit to the assumed distribution, we checked statistical significance using bootstrapped standard errors, based on 1000 bootstrapped samples and randomly assigned seed values. We also ran sensitivity analyses to assess the stability of key results to model specifications: analysing unplanned hospital admission and death as time-to-event variables rather than as binary outcomes, with patients censored on reaching the outcome or loss to follow-up; and analysing change in number of drugs from baseline as a continuous variable, in place of number of drugs at last follow-up as a Poisson count.
In addition to the primary intention-to-treat analysis, we re-ran all analyses with the sample restricted to patients who fully followed the trial protocol. For the per protocol sample we excluded a small number of participants who, although randomised, did not meet the trial criteria of using eight or more drugs at baseline or age 75 or older (n=60) and a larger number who officially withdrew (or were withdrawn when their practice withdrew) before the 24 month follow-up (n=349), or did not attend the 24 month follow-up (n=101).
No adjustment for multiple testing was applied and an α value of 5% was used throughout. Analyses were conducted using Stata V15, with the exception of data derived from the electronic decision support tool (number and types of recommendations generated), which were analysed using SPSS V24.
Monitoring
Staff independent of the research team at each study centre carried out monitoring according to a prespecified protocol. An independent safety and data monitoring committee, masked to randomisation allocation, regularly reviewed the accumulated trial data for safety reasons. However, no concerns arose to terminate the trial.
Patient and public involvement
No patients were involved in setting the research question or developing plans for design of the study, nor were they asked to advise on interpretation or writing up of results.
Results
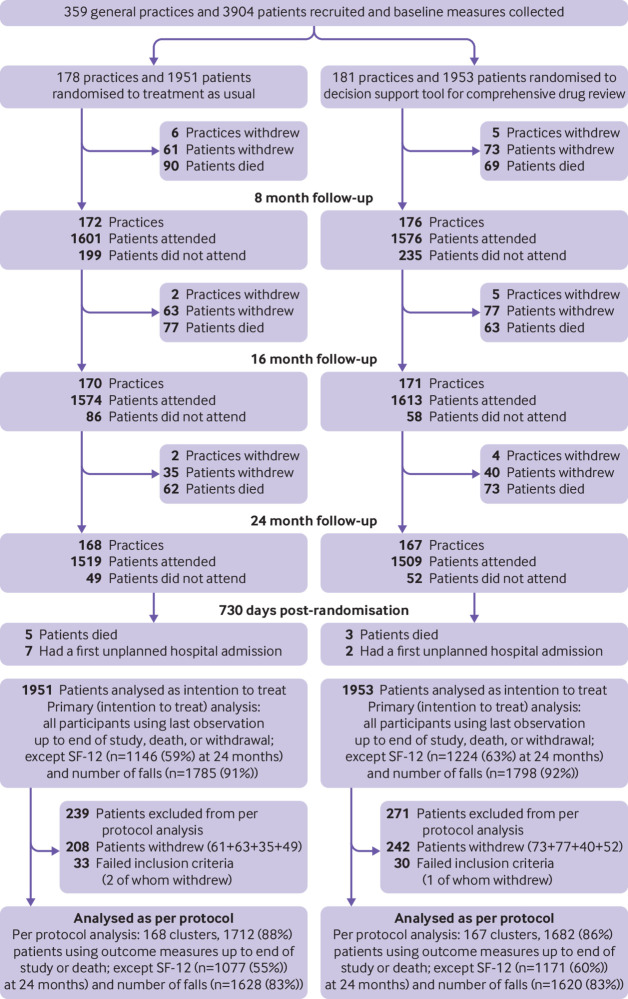
A total of 359 practices recruited 3904 participants. After randomisation, the computerised decision support group consisted of 181 practices and 1953 participants (intervention group) and the treatment as usual group of 178 practices and 1951 patients (control group). The characteristics of the two groups were similar at baseline (table 2). On average, the participants were aged 81.5 (SD 4.4) years, used 10.5 (SD 2.4) drugs, and had 9.5 (SD 4.9) diagnoses; 2240 (57.4%) were women. Small numbers of practices and participants were lost between follow-ups owing to withdrawal or death (fig 1). Final follow-up data were collected for 77% of participants (88% including deaths).
Table 2.
Baseline characteristics of participants with polypharmacy assigned to a computerised decision support tool for comprehensive drug review or treatment as usual (control group). Values are numbers (percentages) unless stated otherwise
| Characteristics | Decision support group (n=1953) | Control group (n=1951) |
|---|---|---|
| Mean (SD) age (years) | 81.5 (4.4) | 81.5 (4.5) |
| Women | 1103 (56.5) | 1137 (58.3) |
| Men | 850 (43.5) | 814 (41.7) |
| Educational level*: | ||
| Low | 788 (40.3) | 748 (38.3) |
| Medium | 739 (37.8) | 726 (37.2) |
| High | 285 (14.6) | 292 (15.0) |
| Missing | 141 (7.2) | 185 (9.5) |
| Smoking status: | ||
| Smoker | 66 (3.4) | 88 (4.5) |
| Former or non-smoker | 1806 (92.5) | 1776 (91.0) |
| Missing | 81 (4.1) | 87 (4.5) |
| Body mass index: | ||
| <18.5 | 15 (0.8) | 19 (1.0) |
| 18.5-24 | 483 (24.7) | 474 (24.3) |
| 25-29 | 816 (41.8) | 790 (40.5) |
| ≥30 | 639 (32.7) | 668 (34.2) |
| Clinical frailty scale†: | ||
| Fit, well, or managing well | 872 (44.6) | 771 (39.5) |
| Vulnerable | 426 (21.8) | 442 (22.7) |
| Mildly frail | 298 (15.3) | 362 (18.6) |
| Moderately frail | 255 (13.1) | 250 (12.8) |
| Severely or very severely frail | 47 (2.4) | 58 (3.0) |
| Missing | 55 (2.8) | 68 (3.5) |
| No of falls in past three months: | ||
| 0 | 1752 (89.7) | 1733 (88.8) |
| 1 | 154 (7.9) | 154 (7.9) |
| ≥2 | 47 (2.4) | 64 (3.3) |
| SF-12 composite scores: | ||
| Physical health | 37.1 (9.4) (n=1774) | 36.7 (9.6) (n=1710) |
| Mental health | 48.1 (11.2) (n=1773) | 48.2 (11.0) (n=1710) |
| Mean (SD) No of drugs | 10.5 (2.5) | 10.5 (2.4) |
| Mean (SD) No of diagnoses | 9.3 (4.4) (n=1952) | 9.7 (5.4) (n=1946) |
| Study centre: | ||
| Bolzano, Italy | 451 (23.1) | 450 (23.1) |
| Manchester, UK | 362 (18.5) | 331 (17.0) |
| Salzburg, Austria | 292 (15.0) | 295 (15.1) |
| Rostock, Germany | 475 (24.3) | 506 (25.9) |
| Witten, Germany | 373 (19.1) | 369 (18.9) |
Fig 1.
Flow of general practices and participants through study
Primary outcome
Of 1953 participants in the intervention group, 871 (44.6%) experienced the primary outcome of unplanned admission to hospital or death by 24 months, and a further 126 (6.5%) were lost to follow-up. Of 1951 participants in the control group, 944 (48.4%) were admitted to hospital or died, and a further 111 (5.7%) were lost to follow-up. In an intention-to-treat analysis (in which participants who were lost to follow-up were included as having reached the outcome) no evidence was found of a difference between the intervention and control groups (odds ratio 0.88, 95% confidence interval 0.73 to 1.07; P=0.19). Sensitivity analysis using either multiple imputation of missing outcomes (P=0.11) or Cox regression (time to unplanned hospital admission or death) supported this finding (hazard ratio 0.93, 95% confidence interval 0.82 to 1.05; P=0.24 (table 3, fig 2). In an analysis of participants who attended practice visits according to protocol only (per protocol analysis), a statistically significant risk reduction favouring the intervention group was observed (odds ratio 0.82, 95% confidence interval 0.68 to 0.98; P=0.03, see supplementary appendix table 1).
Table 3.
Primary and secondary outcomes at last follow-up
| Outcomes | Decision support group | Control group | Adjusted comparison estimate (95% CI) | P value (sensitivities) | |||
|---|---|---|---|---|---|---|---|
| No | Estimate* | No | Estimate* | ||||
| Primary outcome | |||||||
| First unplanned hospital admission or death† | 1953 | 997 (51.0%) | 1951 | 1055 (54.1%) | OR: 0.88 (0.73 to 1.07) | 0.19 (MI‡ 0.114) | |
| Sensitivity: time to first unplanned hospital admission or death | 1953 | 0.46 (0.01)§ | 1951 | 0.50 (0.01)§ | HR: 0.93 (0.82 to 1.05) | 0.24 | |
| Key secondary outcome | |||||||
| Last recorded No of drugs | 1953 | 10.12 (3.01) | 1951 | 10.52 (2.94) | Coefficient¶: 0.95 (0.94 to 0.97) | <0.001 (MI<0.001) (BS<0.001) | |
| Sensitivity: change in No of drugs from baseline | 1953 | −0.42 (2.16) | 1951 | 0.06 (2.04) | MD: −0.45 (−0.63 to -0.26) | <0.001 (MI<0.001) (BS<0.001) | |
| Other secondary outcomes | |||||||
| Death† | 1953 | 380 (19.5%) | 1951 | 366 (18.8%) | OR: 1.01 (0.73 to 1.38) | 0.96 | |
| Sensitivity: time to death | 1953 | 0.11 (0.01)§ | 1951 | 0.12 (0.01) § | HR: 0.90 (0.71 to 1.13) | 0.35 | |
| First unplanned hospital admission† | 1953 | 945 (48.4%) | 1951 | 990 (50.7%) | OR: 0.92 (0.76 to 1.10) | 0.36 | |
| Sensitivity: time to hospital admission | 1953 | 0.49 (0.01)§ | 1951 | 0.52 (0.01)§ | HR: 0.95 (0.83 to 1.07) | 0.38 | |
| No of unplanned hospital admission | 1953 | 0.76 (1.24) | 1951 | 0.87 (1.34) | IRR: 0.91(0.69 to 1.20) | 0.51 (BS 0.351) | |
| Duration of unplanned hospital admission (days) | 1949 | 7.89 (17.43) | 1948 | 8.47 (18.18) | IRR: 0.95 (0.67 to 1.35) | 0.79 (BS 0.707) | |
| No of falls over trial period | 1798 | 0.50 (1.26) | 1785 | 0.51 (1.24) | IRR: 1.08 (0.88 to 1.34) | 0.44 (BS 0.287) | |
| ≥1 fractures during trial period | 1953 | 59 (3.0%) | 1951 | 45 (2.3%) | OR: 1.37 (0.87 to 2.16) | 0.17 | |
| SF-12: | |||||||
| Physical component score (0-100) | 1223 | 36.73 (9.44) | 1146 | 36.32 (9.11) | MD: 0.07 (-0.69 to 0.83) | 0.85 | |
| Mental component score (0-100) | 1224 | 46.66 (11.09) | 1145 | 46.27 (11.18) | MD: 0.34 (-0.69 to 1.37) | 0.52 | |
OR=odds ratio; MI=multiple imputation; HR=hazard ratio; BS=bootstrap; MD=mean difference; IRR=incidence rate ratio.
Mean (standard deviation) or number (%) unless otherwise indicated.
Participants who dropped out were analysed as having reached the endpoint.
Participants who dropped out were analysed using multiple imputation.
Estimated proportion (standard error) reaching endpoint by 24 months, from survivor function.
Coefficient represents the adjusted ratio of the number of prescribed drugs in participants assigned to electronic decision support versus those assigned to treatment as usual (control group).
Fig 2.
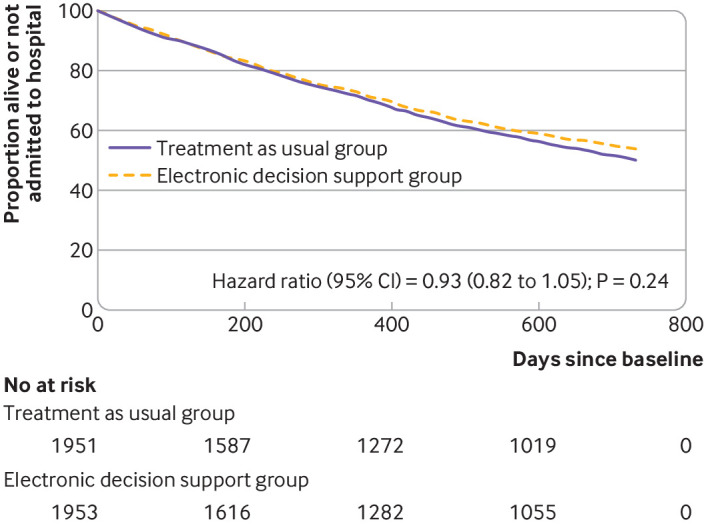
Kaplan-Meier survival plot of time to death or first unplanned hospital admission for participants assigned to an electronic decision support tool or treatment as usual (control group): results from intention-to-treat analysis
Secondary outcomes
The key secondary outcome of number of drugs at final follow-up was lower in the intervention than control arm (table 3: incidence rate ratio 0.95 (95% confidence interval 0.94 to 0.97; P<0.001). Sensitivity analysis using the change in total number of drugs from baseline as a continuous measure supported this result: the uncontrolled mean reduction was −0.42 (95% confidence interval −0.58 to −0.27) in the intervention group compared with 0.06 (−0.06 to 0.18) in the control group, and the controlled mean difference was −0.45 (−0.63 to −0.26; P<0.001). The results remained unchanged after further sensitivity analysis using multiple imputation and bootstrapping, and in the per protocol analysis.
No overall statistically significant differences were found between trial arms for the secondary outcomes of time to first unplanned hospital admission or time to death as single outcomes, number or duration of hospital admissions, number of falls, fractures, or SF-12 physical and mental component scores. Sensitivity analysis using bootstrapping when relevant did not alter the results for these outcomes.
The total number of recommendations elicited by the electronic decision support tool (not visible to the doctors in the control group) to adjust or eliminate potentially inappropriate drugs did not differ between the two trial groups at baseline. Owing to changes in drugs during the trial, the number of recommendations decreased in both groups but the reduction at the final visit compared with baseline was larger in the intervention group (control group −2.43, 95% confidence interval −2.61 to −2.25; intervention group −3.38, −3.56 to −3.20) (see supplementary appendix table 2). Appendix tables 2-9 show more results on the number and types of drugs deprescribed.
In per protocol analysis (numbers were reduced compared with the per protocol analysis of the primary endpoint as a result of missing data), we also compared the presence and number of symptoms or possibly adverse drug reactions between the control and intervention groups at baseline and at the final visit. Mean numbers of symptoms were similar between the groups at baseline. The number of symptoms declined in both groups over the trial by similar amounts and did not differ at 24 months (P=0.34) (see supplementary appendix tables 10-12).
Use of the electronic decision support tool in the intervention group was monitored by counting the number of new datasets for each participant created during the study. A new dataset was recorded each time a participant’s drug was changed or a study visit was made. The doctors in the intervention group created 18.7 (SD 8.8) datasets for each participant throughout the study, whereas doctors in the control group created only 12.1 (SD 5.1) datasets.
Discussion
This large randomised controlled trial of elderly people (≥75 years) with polypharmacy investigated the effects of a decision support tool for comprehensive drug review provided electronically to doctors. No conclusive evidence was found that first unplanned admission to hospital or all cause mortality differed between the intervention and treatment as usual (control) groups, but the number of drugs was reduced in the intervention group.
Even though we found no conclusive evidence in the intention-to-treat analysis for an effect on the primary outcome, the upper 95% confidence interval of the odds ratio of 1.07 (which corresponds to an estimated 3% increase in risk34) makes it unlikely that the reduction in drugs prompted by the decision support tool leads to any appreciable increase in number of hospital admissions or deaths, as does the statistically significant reduction in hospital admissions or deaths among participants visiting their general practice according to protocol. Taken together, the results suggest that the number of drugs was reduced without increasing harm to patients, which is a positive result in itself.35
It might be possible that the two year observation period was too short to observe a clear positive effect. This is also suggested by the Kaplan-Meier survival plot of combined hospital admissions and deaths, as the curves for both treatment groups split after about a year and gradually move apart. Although the degree of deprescribing appears modest (on average 0.45 drugs from a baseline of 10.5), at the population level this would represent a substantial reduction in overall treatment burden and in prescribing costs. That deprescribing was directly related to use of the electronic decision support tool is supported by the corresponding reduction in the number of recommendations appearing in follow-up drug reviews for intervention participants compared with controls, which in itself implies improvement of drug appropriateness.
Strengths and weaknesses of this study
We postulate that the results of our large randomised controlled trial are generalisable to the wider elderly population. Since most of the participants were not frail, however, our results might not translate so well to a frailer population.
Another strength of our trial was the two year observation period, which is longer than in most other trials in this specialty. The pragmatic nature of the trial provides real world evidence of a clinically relevant intervention. That the electronic decision support tool was not always used as intended, as shown by our process evaluation,10 11 reflects daily clinical practice. However, this reduces potential effectiveness of the tool, which might be reflected by the differing result between our intention-to-treat and per protocol analyses.
The lack of blinding of doctors and patients because of the nature of the trial could be considered a weakness. However, we collected baseline data before randomisation to assure allocation concealment, and we blinded all statisticians involved in data analysis to minimise bias.
Also, selection bias might have occurred when doctors identified participants. We advised the doctors to select a random sample of eligible patients, but we were not able to verify this owing to the pragmatic nature of the trial. Besides, not all doctors followed the instructions to include 11 patients—recruitment varied from 1 to 30 participants per practice.
As our trial was international it has the advantage that we were able to test the electronic decision support tool in various healthcare settings, thus increasing generalisability. Important differences do, however, exist between the participating countries. Italy and the UK have a primary care centred healthcare system, hence although we standardised instructions for the recording of data, Italian and British doctors might have had a better overview of all drug data and diagnoses compared with German and Austrian doctors. Any impact of this should be balanced between groups by the stratified randomisation within research centres, and we adjusted for centre effects in the analysis.
In addition, true drug consumption is difficult to assess: we instructed doctors to ask their patients about all current drugs, but we were not able to verify actual drug consumption.
We used a cluster design to avoid contamination of the treatment as usual group. However, control doctors were aware of the purpose of the trial and this, together with regular completion of the electronic case report form plus any additional patient consultations, might have resulted in more changes to drugs among controls than under typical usual care. Any effects of this on outcomes would have been conservative in that it would have tended to reduce differences between the treatment groups.
We were not able to assess whether drug related hospital admissions or other drug specific adverse events were reduced; however, drugs often associated with preventable hospital admissions such as antiplatelets or diuretics36 were among the commonly deprescribed drugs in the electronic decision support group.
A weakness of the decision support tool is that it cannot deal with individual patient needs. The tool is intended to support doctors and provide additional useful information that would otherwise be time consuming to obtain. It will never be able to replace doctor care as it relies entirely on data that are encoded and extracted from the patient record. It cannot consider patient preferences. For optimal drug treatment it is therefore important that doctors discuss the recommendations provided by the decision support tool in a shared decision making process with patients. Although we asked doctors in our trial to use the decision support tool as instructed, our process evaluation showed that not all doctors complied. This probably led to a lower adherence to the recommendations of the tool.
Comparison with other studies
Previous research on the appropriate use of drugs in people with polypharmacy consists of a range of interventions in different settings using diverse study designs and outcomes. Few interventions have provided electronic decision support to doctors in their own practice.4 Although several studies have shown some positive effects, mainly on drug appropriateness, in general these interventions had little or no effect on hospital admission4 or all cause mortality.5 Our study also did not find a conclusive effect on these outcomes, but per protocol analysis and the Kaplan-Meier-curve make it likely that proper use of the decision tool for a longer period could be beneficial.
Despite different definitions of number of drugs used and considerable heterogeneity in results, a systematic review of polypharmacy interventions estimated a mean reduction in drug numbers of 0.2 under the intervention but an increase of 0.2 under the control condition.5 This is consistent with our finding of a reduction of 0.45 between groups.
Implications of the findings and suggestions for future research
We postulate that the evidence derived from this study is strong enough to support implementation of the electronic decision support tool for comprehensive drug review on a wider scale. Furthermore, efforts at the policy level are needed to provide resources to use the tool in daily practice routine, discuss the recommendations of the tool with patients in a shared decision making process, and deprescribe inappropriate drugs during regular consultations. Eventually, this should lead to an improved integration of the support tool into doctors’ workflow.
Future efforts should focus on integrating the decision support tool into the electronic health records used by doctors, as the extra entry of patient data into the electronic case report form is time consuming.11 Consideration should also be given to adding drug start recommendations to the electronic decision support tool to avoid underuse. Widespread implementation of an adapted version of the tool should be accompanied by research to assess intended and unintended effects in daily routine practice.
Conclusions
Although no conclusive evidence for the reduction of a composite of unplanned hospital admission or death was found within the follow-up period of two years, a reduction in prescribing was achieved without detriment to patient outcomes. We feel this evidence is strong enough to support implementation of the electronic decision support tool on a wider scale, preferably integrated within electronic health records.
What is already known on this topic
Polypharmacy is common in older people, often inappropriate, and associated with potential harms, yet no ideal strategy for optimising prescribing exists
A Cochrane review concluded that it remains unclear whether interventions to improve prescribing in patients with polypharmacy enhance drug appropriateness or decrease potentially inappropriate prescribing or the proportion of patients with one or more potentially inappropriate drugs
The reviewed interventions made little or no difference to hospital admissions but might have slightly reduced potential prescribing omissions
What this study adds
A computerised decision support tool for comprehensive drug review in elderly patients (≥75 years) with polypharmacy resulted in a reduction in inappropriate drugs
Although no difference in unplanned hospital admission or death was found over two years in intention-to-treat analysis, the reduction in drugs was achieved without detriment to patient outcomes
Acknowledgments
We thank the participating doctors and patients; the PRIMA-eDS team for their support in conducting the trial—in particular, Annette Barber, Vicky Bell, Barbara Faller, Jennifer Höck, Anna Renom-Guiteras, Sophie Keller, Celine Kriechmayr, Joonas Mäkinen, Yolanda Martinez, Peter Nyberg, Giulio Pirolo, Angela Swallow, Anne-Lisa Teichmann, Sabine Weißbach, and Martina Valentini; the SVEMG (Società Veneta di Medicina Generale) for its support in recruiting doctors in Italy; and the five National Institute for Health Research Clinical Research Networks that supported recruitment in the UK (Eastern, North Eastern, Wales, North West Coast, and Greater Manchester).
Web extra.
Extra material supplied by authors
Supplementary information: online appendix
Contributors: AS (primary investigator), MF, IK, and AE conceptualised the development of the electronic decision support tool and the PRIMA-eDS project. AA, AV, AW, CL, DR, and GP contributed to the refinement of methodology and local realisation of the trial. AS and AR led the PRIMA-eDS project and the trial. MF and TJ were responsible for the trial at the study centre Salzburg. ED was responsible for the trial at the study centre Rostock, supported by AA and CL who were responsible for quality assurance. GP was responsible for the trial in Italy. UT and CS supported the conduct of the trial at the study centre Witten/Herdecke, AV in Italy, and AW in the UK. DR and RKM were responsible for the sample size calculation, statistical analysis plan, and statistical evaluation. MH carried out the statistical evaluation. AR, AS, and DR conceptualised this manuscript. All authors read and approved the final manuscript. AS is the lead author and the manuscript’s guarantor. AR attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by the seventh Framework Programme of the European Union, theme Health-2012-Innovation-1-2.2.2-2 (grant agreement No 305388-2). The funder had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the article for publication. All authors confirm that they worked independently from funders. All authors had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: This study was funded by the seventh Framework Programme of the European Union, theme Health-2012-Innovation-1-2.2.2-2 (grant agreement No 305388-2). All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. IK is a salaried employee of Duodecim Medical Publications, a company that develops and sells the EBMeDS (evidence based medicine electronic decision support) service that was used as the technology platform of the intervention in the trial. All other authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the five local ethics committees: Ethikkommission der Universität Witten/Herdecke, 3 December 2013 (103/2013), NRES Committee North West Greater Manchester East, 6 June 2014 (14/NW/0197), Ethikkommission für das Bundesland Salzburg, 15 September 2013 (08.04.2014 (415-E/1509/20-2014)), Ethikkommission der Universitätsmedizin Rostock, 3 February 2014 (A 2014-0020), and Comitato etico di Belluno (Azienda ULSS), 19 June 2013 (305388-2).
Data sharing: Data from the study or the trial materials are available on reasonable request from the principal investigator at andreas.soennichsen@meduniwien.ac.at. Proposals requesting data access will need to specify how it is planned to use the data.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Lay information of the results of the trial will be sent to all participating doctors by email or letter once the results of the trial are published. Lay information of the key results of the study will be made available on the PRIMA-eDS homepage (www.prima-eds.eu).
References
- 1. Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995-2010. BMC Med 2015;13:74. 10.1186/s12916-015-0322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bokhof B, Junius-Walker U. Reducing Polypharmacy from the Perspectives of General Practitioners and Older Patients: A Synthesis of Qualitative Studies. Drugs Aging 2016;33:249-66. 10.1007/s40266-016-0354-5 [DOI] [PubMed] [Google Scholar]
- 3. Wilson T, Buck D, Ham C. Rising to the challenge: will the NHS support people with long term conditions? BMJ 2005;330:657-61. 10.1136/bmj.330.7492.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rankin A, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2018;9:CD008165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johansson T, Abuzahra ME, Keller S, et al. Impact of strategies to reduce polypharmacy on clinically relevant endpoints: a systematic review and meta-analysis. Br J Clin Pharmacol 2016;82:532-48. 10.1111/bcp.12959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez YV, Renom-Guiteras A, Reeves D, et al. A set of systematic reviews to help reduce inappropriate prescribing to older people: study protocol. BMC Geriatr 2017;17(Suppl 1):231. 10.1186/s12877-017-0570-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010;170:1648-54. 10.1001/archinternmed.2010.355 [DOI] [PubMed] [Google Scholar]
- 8. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Isr Med Assoc J 2007;9:430-4. [PubMed] [Google Scholar]
- 9. Sönnichsen A, Trampisch US, Rieckert A, et al. Polypharmacy in chronic diseases-Reduction of Inappropriate Medication and Adverse drug events in older populations by electronic Decision Support (PRIMA-eDS): study protocol for a randomized controlled trial. Trials 2016;17:57. 10.1186/s13063-016-1177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieckert A, Teichmann A-L, Drewelow E, et al. Reduction of inappropriate medication in older populations by electronic decision support (the PRIMA-eDS project): a survey of general practitioners’ experiences. J Am Med Inform Assoc https://academic.oup.com/jamia/advance-article-pdf/doi/10.1093/jamia/ocz104/29521023/ocz104.pdf. [DOI] [PMC free article] [PubMed]
- 11. Rieckert A, Sommerauer C, Krumeich A, Sönnichsen A. Reduction of inappropriate medication in older populations by electronic decision support (the PRIMA-eDS study): a qualitative study of practical implementation in primary care. BMC Fam Pract 2018;19:110. 10.1186/s12875-018-0789-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rieckert A, Trampisch US, Klaaßen-Mielke R, et al. Polypharmacy in older patients with chronic diseases: a cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam Pract 2018;19:113. 10.1186/s12875-018-0795-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evidence-Based Medicine Guideline. https://www.duodecim.fi/english/products/ebmg Accessed May 03, 2018.
- 14.Evidence-Based Medicine electronic Decision Support. https://www.ebmeds.org/web/guest/home? Accessed May 03, 2018.
- 15.EBMeDS Clinical Decision Support. http://www.ebmeds.org Accessed May 03, 2018.
- 16. Sommerauer C, Schlender L, Krause M, et al. Correction to: Effectiveness and safety of vitamin K antagonists and new anticoagulants in the prevention of thromboembolism in atrial fibrillation in older adults - a systematic review of reviews and the development of recommendations to reduce inappropriate prescribing. BMC Geriatr 2018;18:12. 10.1186/s12877-017-0663-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meinshausen M, Rieckert A, Renom-Guiteras A, et al. Effectiveness and patient safety of platelet aggregation inhibitors in the prevention of cardiovascular disease and ischemic stroke in older adults - a systematic review. BMC Geriatr 2017;17(Suppl 1):225. 10.1186/s12877-017-0572-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schlender L, Martinez YV, Adeniji C, et al. Efficacy and safety of metformin in the management of type 2 diabetes mellitus in older adults: a systematic review for the development of recommendations to reduce potentially inappropriate prescribing. BMC Geriatr 2017;17(Suppl 1):227. 10.1186/s12877-017-0574-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schott G, Martinez YV, Ediriweera de Silva RE, et al. Effectiveness and safety of dipeptidyl peptidase 4 inhibitors in the management of type 2 diabetes in older adults: a systematic review and development of recommendations to reduce inappropriate prescribing. BMC Geriatr 2017;17(Suppl 1):226. 10.1186/s12877-017-0571-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sommerauer C, Kaushik N, Woodham A, et al. Thiazides in the management of hypertension in older adults - a systematic review. BMC Geriatr 2017;17(Suppl 1):228. 10.1186/s12877-017-0576-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vögele A, Johansson T, Renom-Guiteras A, et al. Effectiveness and safety of beta blockers in the management of hypertension in older adults: a systematic review to help reduce inappropriate prescribing. BMC Geriatr 2017;17(Suppl 1):224. 10.1186/s12877-017-0575-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Renom-Guiteras A, Meyer G, Thürmann PA. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol 2015;71:861-75. 10.1007/s00228-015-1860-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renbase - Analysis of adverse drug reactions. http://www.medbase.fi/en/professionals/renbase Accessed May 03, 2018.
- 24.Interaction Database INXBASE. http://www.medbase.fi/en/professionals/inxbase Accessed 3 May 20108.
- 25.European Medicines Agency. https://www.ema.europa.eu/ema Accessed May 03, 2018.
- 26.RISKBASE – Analysis of adverse drug reactions. Available at: http://www.medbase.fi/en/professionals/riskbase Accessed May 03, 2018.
- 27.World Health Organization. The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD). https://www.who.int/classifications/atcddd/en/ Accessed February 14, 2018.
- 28.World Health Organization. Classification of Diseases (ICD). https://www.who.int/classifications/icd/en/ Accessed February 14, 2018.
- 29. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489-95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maruish MEE. User’s manual for the SF-12v2 Health Survey. 3rd ed. QualityMetric: Lincoln, RI.
- 31. OECD Classifying Educational Programmes – Manual for ISCED-97 Implementation in OECD Countries –. 1999 Edition OECD, 1999. [Google Scholar]
- 32. Sönnichsen AC, Winkler H, Flamm M, et al. The effectiveness of the Austrian disease management programme for type 2 diabetes: a cluster-randomised controlled trial. BMC Fam Pract 2010;11:86. 10.1186/1471-2296-11-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sullivan TR, White IR, Salter AB, Ryan P, Lee KJ. Should multiple imputation be the method of choice for handling missing data in randomized trials? Stat Methods Med Res 2018;27:2610-26. 10.1177/0962280216683570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690-1. 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 35. Reeve E, Thompson W, Farrell B. Deprescribing: A narrative review of the evidence and practical recommendations for recognizing opportunities and taking action. Eur J Intern Med 2017;38:3-11. 10.1016/j.ejim.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 36. Howard RL, Avery AJ, Slavenburg S, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2007;63:136-47. 10.1111/j.1365-2125.2006.02698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: online appendix