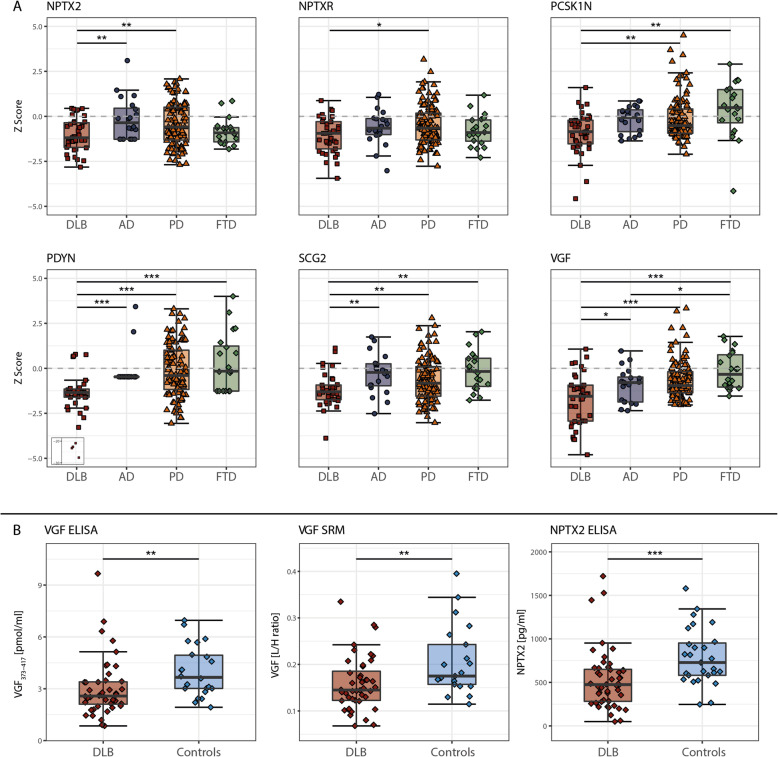
Fig. 5.
Validation of candidate biomarkers. a Differences in levels of candidate biomarkers between DLB and related neurodegenerative diseases. All protein levels were Z transformed according to the mean and standard deviation in controls, dotted line represents average protein levels for the control group. For PDYN, 4 outliers (z-score > 20) were illustrated in a box. Please note that the low variation in PDYN levels in AD patients is caused by lack of a measurable concentration.Differences were assessed with GLM corrected for age and a FDR correction was applied. * p < 0.05, ** p < 0.01, *** p < 0.001. b Validation of VGF and NPTX2 using orthogonal analytical methods. Levels of VGF373–417 (pmol/ml) were determined by ELISA, levels of VGF [LH/ratio] were determined with SRM and levels of NPTX2 (pg/ml) were determined with ELISA in CSF samples from DLB patients (n = 48) and controls (n = 28). The line through the middle of the boxes corresponds to the median and the lower and the upper lines to the 25th and 75th percentile, respectively. The whiskers extend from the 5th percentile on the bottom to the 95th percentile on top. Differences between DLB patients and controls were assessed with GLM. * p < 0.05, ** p < 0.01, *** p < 0.001. Abbreviations: AD, Alzheimer’s disease; DLB, Dementia with Lewy bodies; ELISA, enzyme-linked immunosorbent assays; FTD, frontotemporal dementia; NPTX2, Neuronal pentraxin 2; NPTXR, Neuronal pentraxin receptor, PCSK1N, ProSAAS; PD, Parkinson’s disease; PDYN, Proenkephalin-B; SCG2, Secretogranin-2; SRM, selected reaction monitoring; VGF, Neurosecretory protein VGF