Abstract
Recently, chemical modifications of chitosan (CS) have attracted the attention of scientific researchers due to its wide range of applications. In this research, chitin (CH) was extracted from the scales of Cyprinus carpio fish and converted to CS by three chemical steps: (i) demineralization, (ii) deprotonation, and (iii) deacetylation. The degree (measured as a percentage) of deacetylation (DD %) was calculated utilizing the acid–base titration method. The structure of CS was characterized by Fourier transform infrared (FT-IR) spectroscopy and thermogravimetric analysis (TGA). Three new CS Schiff bases (CSSBs) (CS-P1, CS-P2, and CS-P3) were synthesized via coupling of CS with 2-chloroquinoline-3-carbaldehyde, quinazoline-6-carbaldehyde, and oxazole-4-carbaldehyde, respectively. The newly prepared derivatives were verified, structurally, by nuclear magnetic resonance (1H and 13C NMR) and FT-IR spectroscopy. Antimicrobial activity was evaluated for the prepared compounds against both “Gram-negative” and “Gram-positive” bacteria, namely, Escherichia coli, Klebsiella pneumonia, Staphylococcus aureus, and Streptococcus mutans, in addition to two kinds of fungi, Candida albicans and Aspergillus fumigates. Cytotoxicity of the synthesized CSSBs was evaluated via a MTT screening test. The results indicated a critical activity increase of the synthesized compound rather than CS generally tested bacteria and fungi and the absence of cytotoxic activity. These findings suggested that these new CSSBs are novel biomaterial candidates with enhanced antibacterial and nontoxic characteristics for applications in areas of both biology and medicine.
Keywords: Amino Polysaccharide, Chitin, Chitosan, Chitosan Schiff base, Antimicrobial polymer, Cytotoxicity test
1. Introduction
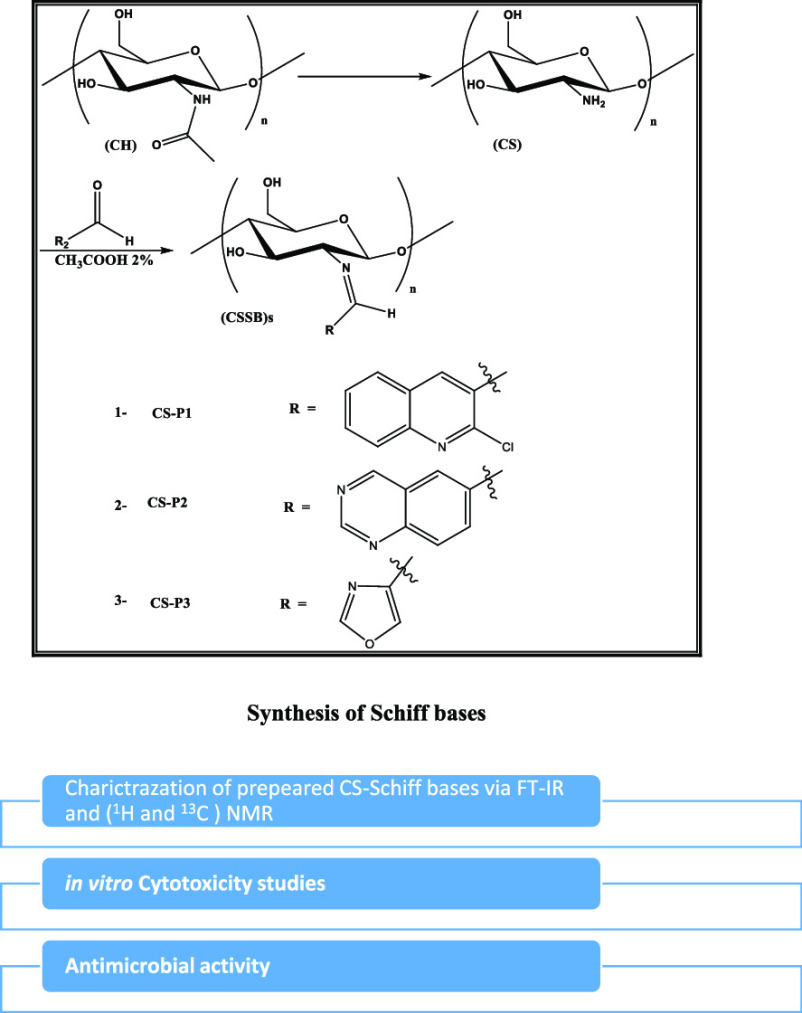
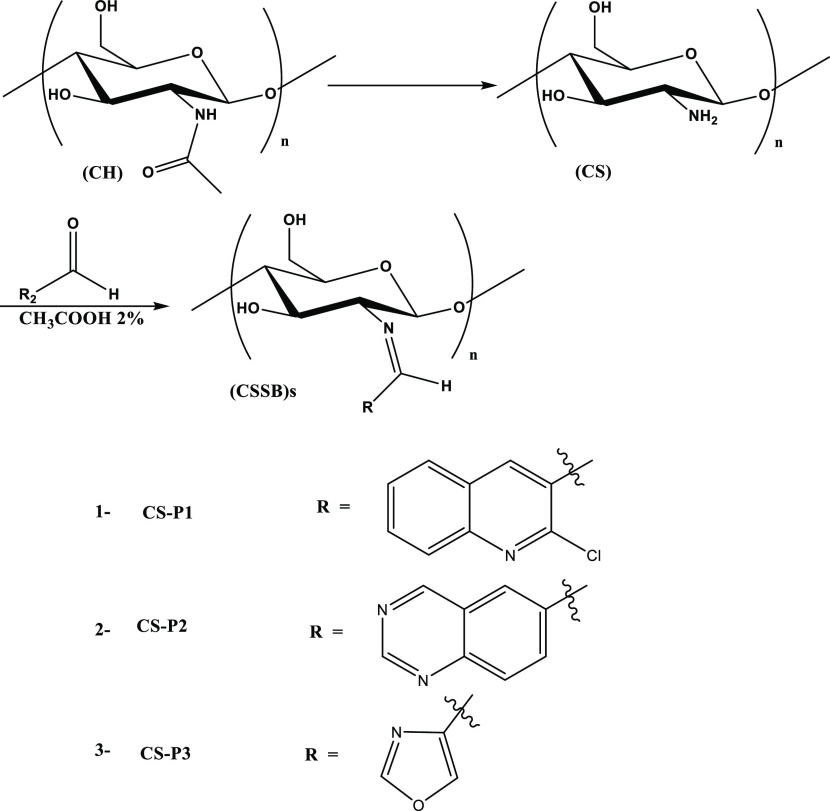
CS has a chemical structure of α(1→4)-2-amino-2-deoxy-β-d-glucopyranose derived from the N-deacetylation of CH, a typical auxiliary biopolymer found in the exoskeletons of roaches and crustaceans and fungi cell walls (Figure 1). The main source of CH and CS is shellfish, for example, crabs, shrimp, and lobsters, and fish scales. It has a few receptive amino groups, which offer further chemical modifications for the development of an incredible assorted variety of valuable derivatives that are cost-effective.1 CS, deacetylated CH, is an extremely useful, readily available bioactive polymer, which is a renewable, natural, nontoxic, edible, and biodegradable polymer characterized by biocompatibility.2,3 CS shows several advantageous biological properties, such as antitumor, antimicrobial, and hemostatic activities, and promotes wound healing.4 It has versatile applications ranging from biomedical designing, pharmaceuticals, drug transport, restorative materials, metal particle chelation, and absorptivity to water treatment and plant security.5−7 CS derivatives, especially those synthesized via a Schiff base reaction, are the most important due to their organic application characteristics. Recently, the reaction of CS with the rings of aromatic and heterocyclic aldehydes resulted in the efficient production of stable Schiff bases (SBs), which are exceptional compounds in many application areas, particularly in pharmacology and medicine, e.g., as antimicrobial and cancer prevention agents.8,9 CSSBs are characteristically prepared by the superficial reaction of CS amine sites with aldehydes or ketones by the elimination of water particles.10
Figure 1.
Structure of CH and Cyprinus carpio fish.
Furthermore, quinoline and quinazoline compounds are present in several natural products and in manufactured pharmacologically significant heterocyclic materials. Quinoline and quinazoline derivatives are called antimalarial,11,12 antiviral,13,14 antibacterial,15,16 analgesic,17,18 antihepatoma,19,20 and anti-inflammatory agents.21,22 Also oxazole derivatives are known as one of the most essential kinds of heterocyclic compounds, which are very significant for medicinal chemistry.23
In this study, based on the above facts, first, CH is extracted from the local Cyprinus carpio fish scales by demineralization and deprotonation followed by deacetylation to produce CS using fresh reagents, thereby reducing the time for the overall procedure to obtain CS with a degree of deacylation (DD) percentage of at least 60. Second, this study aims to determine the DD % of the products; samples of CS are obtained in different steps of the procedure by the acid–base titration method. Third, this study also aims to characterize and verify the physicochemical properties of the products employing FT-IR and TGA. Finally, this study attempts to develop a synthesis method for three new CSSB derivatives CS-P1, CS-P2, and CS-P3 through coupling CS with 2-chloroquinoline-3-carbaldehyde, quinazoline-6-carbaldehyde, and oxazole-4-carbaldehyde, respectively. The structures of the prepared derivatives were verified via FT-IR and 1H and 13C NMR spectroscopy. The antimicrobial potential of CS and the new derivatives was tested against four kinds of bacteria, E. coli, K. pneumonia, S. aureus, and S. mutans, in addition to two kinds of fungi, C. albicans and A. fumigates. The cytotoxicity of the newly prepared compounds was evaluated through the MTT assay. The results were compared with those of CS.
2. Results and Discussion
Drug resistance of microorganisms to antibiotics has encouraged researchers to search for new antibiotics to challenge this dangerous phenomenon. Many CS derivatives were synthesized to improve the antimicrobial effectiveness of CS, for example, SBs, via methylation, amination, etc.24,25 In the present study, CS was extracted and used to prepare three new SBs of CS by reacting CS with 2-chloroquinoline-3-carbaldehyde, quinazoline-6-carbaldehyde, and oxazole-4-carbaldehyde. The structures of the prepared CSSBs were confirmed using 1H NMR, 13C NMR, and FT-IR techniques.
2.1. CS Extraction Description
The details of the treatment of C1, C2, C3, and C4 samples are presented in Table S1 in the Supporting Information (SI).
2.1.1. Acid–Base Titration Method
The DD % for sample C4 was determined by the acid–base titration method. The DD % was calculated using the endpoint of the acid–base titration when the indicator changed into blue-green colors. The DD % values of the deacetylated products were calculated according to the following equations26,27
| 1 |
| 2 |
where C1 and V1 are the concentration and the volume of HCl used, respectively, C2 and V2 are the concentration and the amount of NaOH used for titration, respectively, W is the weight of samples used for acid–base titration. The calculated DD for each sample is tabulated in Table S2 and Figure S1 in the SI.
2.1.2. FT-IR Spectroscopic Study
FT-IR spectroscopy was used to study the structures of CH and the related derivatives. The FT-IR spectra of CS, CH, C3, and C4 are shown in Figures S2–S5 in the SI, respectively. By comparing the spectra for sample C3 and CH, and sample C4 and CS, several bands in the range of 4000–400 cm–1 were noted in the spectra. As shown in Table S3 in the SI, bands of the synthesized CS match with those of CH. The primary amines showed various bands in the range 3425–2881 cm–1 correlated with ν(N–H) and ν(NH2) vibrations. The band at 2921–2879 cm–1 was observed corresponding to the methyl group in pyranose. The appearance of a band at 1597 cm–1 and the disappearance of a band at 1655 cm–1 indicate the deacetylation of CH. The doublet form of the amide band is attributed to the existence of intermolecular and intramolecular hydrogen bonds (C=O···HN, C=O···HOCH2).28−30 The bands at 1264 and 1157 cm–1 are related to the vibrations of NHCO. The bands in the range of 1000–1158 cm–1 were related to the vibrations of C–O–C, C–OH, and C–C ring bonds.31 The characteristic band at 1600–1400 cm–1 due to the N–H bending of CS was observed, in addition to the bands at 1600.9 and 1647.19 cm–1 corresponding to the bending of −NH2.32,33 The existence of CH3, CH2, and CH groups was confirmed by the appearance of vibration peaks within the 1422–603 cm–1 range.
2.1.3. Thermal Gravimetric Analysis (TGA)
TGA of CS, CH, C3, and C4 was performed, as shown in Figures S6–S9 in the SI, respectively. Physicochemical properties of C3 and C4 were compared with those of CH and CS. The thermal stability of C3 was similar to that of CH.
2.2. Preparation of CS Schiff Base Derivatives
Three CSSBs were prepared, as shown in the schematic diagram in Scheme 1. Extracted CS was dissolved in 2.0% aqueous acetic acid, followed by addition of an equimolar amount of the carbonyl compounds dissolved in ethanol to obtain CSSBs. The resultant compounds obtained from the reaction of CS with 2-chloroquinoline-3-carbaldehyde, quinazoline-6-carbaldehyde, and oxazole-4-carbaldehyde were labeled CS-P1, CS-P2, and CS-P3, respectively.
Scheme 1. Preparation of CSSB Derivatives.
2.2.1. Characterization of CSSBs
2.2.1.1. FT-IR Spectroscopy and 1H NMR and 13C NMR Analyses
The FT-IR spectra of CSSBs (CS-P1, CS-P2, and CS-P3) are shown in Figures S10–S12 in the SI, respectively. All spectra of the new derivatives displayed a vibration band at 1633–1655 cm–1 corresponding to the (−C=N) group. The aromatic ring showed a stretching vibration band ranging from 1400 to 1500 cm–1 related to the C–C bond, while the absorption band at 1057 cm–1 corresponded to the aromatic in-plane C–H bending.34 No band was observed in the region 1660–1730 cm–1, which proved the absence of the carbonyl group, which in turn indicated that no residue of free aldehydes remained. The vibration bands at 2921 and 2883 cm–1 were related to the C–H stretching of methyl and methylene groups, respectively.34 The glycosidic bonds showed bands at 1155 and 900 cm–1. The vibration bands at 1205–975 cm–1 were related to the C–O, C–C, and C–O–C stretching of glycosidic bonds and the pyranose ring.35
The structure of the prepared CSSBs was confirmed by 1H and 13C NMR. The 1H NMR spectra of the synthesized derivatives CS-P1, CS-P2, and CS-P3 are shown in Figures S13–S15 in the SI, respectively. The 13C NMR spectra for CS-P1, CS-P2, and CS-P3 are shown in Figures S16–S18 in the SI, respectively. The 1H and 13C NMR data of all SBs are shown below.
2.2.1.2. Compound CS-P1
δH (500 MHz, d6-DMSO) 7.33 (1H, d, J 0.8), 6.84 (1H, dd, J 7.5, 1.4), 6.78 (1H, td, J 7.5, 1.4), 6.38 (1H, td, J 7.5, 1.4), 6.33 (1H, dd, J 7.5, 1.5), 6.12 (1H, s), 5.83 (1H, d, J 2.5), 5.04 (1H, d, J 0.8), 4.93 (1H, s), 4.25 (1H, s), 3.92 (1H, s), 3.62 (1H, td, J 7.7, 4.0), 3.46 (1H, dd, J 12.2, 6.0), 3.35 (1H, ddd, J 9.4, 5.9, 3.5), 3.21 (1H, dd, J 12.3, 5.9), 3.00 (1H, dd, J 4.0, 2.5), 1.80 (1H, ddd, J 12.3, 7.7, 3.5), 1.31 (1H, ddd, J 12.3, 7.7, 3.5).
δC (125 MHz, d6-DMSO)153.38, 143.12, 134.23, 131.24, 129.33, 127.75, 122.07, 120.77, 114.44, 95.68, 74.70, 73.70, 69.45, 66.03, 37.86.
2.2.1.3. Compound CS-P2
δH (500 MHz, d6-DMSO) 7.84 (1H, s), 7.64 (1H, s), 7.30 (1H, s), 7.25 (1H, dd, J 7.5, 1.5), 6.45 (1H, d, J 7.5), 6.14 (1H, d, J 3.1), 5.67 (1H, s), 5.53 (1H, s), 5.26 (2H, s), 3.88 (1H, td, J 7.8, 5.0), 3.64 (1H, s), 3.37 (1H, dd, J 12.4, 1.3), 3.25 (1H, ddd, J 4.8, 3.4, 1.3), 3.11 (1H, dd, J 12.4, 1.3), 2.89 (1H, dd, J 5.0, 3.1), 1.70 (1H, ddd, J 12.3, 7.8, 3.5), 1.30 (1H, ddd, J 12.3, 7.8, 3.5).
δC (125 MHz, d6-DMSO) 164.71, 147.34, 147.27, 139.42, 132.73, 125.45, 121.55, 112.39, 96.94, 75.14, 74.96, 70.71, 67.29, 63.16, 39.52, 39.12.
2.2.1.4. Compound CS-P3
δH (500 MHz, d6-DMSO) 7.36 (1H, d, J 0.8), 6.38 (1H, d, J 2.5), 5.72 (1H, s), 5.26 (1H, s), 4.56 (1H, d, J 0.8), 4.33 (1H, s), 4.18–3.99 (2H, m), 3.89 (1H, s), 3.63 (1H, dd, J 12.4, 1.3), 3.52 (1H, ddd, J 4.9, 3.5, 1.3), 3.38 (1H, dd, J 12.4, 1.3), 3.16 (1H, dd, J 4.4, 2.5), 1.96 (1H, ddd, J 12.3, 7.8, 3.6), 1.54 (1H, ddd, J 12.3, 7.8, 3.6).
δC (125 MHz, d6-DMSO) 158.92, 152.49, 134.20, 97.34, 86.33, 75.36, 71.11, 67.70, 64.24, 39.52, 34.06.
The synthesized new CSSBs presented typical peaks of the Cs and SB parts.
2.2.1.5. Solubility Study
Different organic solvents were used to test the solubility of the synthesized compounds. Table 1 shows the results. The prepared compounds dissolve in dimethyl sulfoxide and mixtures of equal proportions of dimethyl sulfoxide and trifluoroacetic acid. Partial dissolution or swelling was observed in some solvents such as dilute hydrochloric acid and acetic acid at 70 °C. In contrast, the products are not soluble in most inorganic solvents.
Table 1. Solubility Characteristics of CSSBs in a Variety of Solventsa.
| solvents |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CH3COOH |
CF3COOH |
DMSO | HCl | NaOH | H2O | KOH | DMSO + CF3COOH | |||
| comp. codes | 25 °C | 70 °C | 25 °C | 70 °C | 25 °C | 25 °C | 25 °C | 25 °C | 25 °C | 25 °C |
| CS-P1 | S+ | S* | S** | S** | S | S* | S+ | S+ | S** | S |
| CS-P2 | S+ | S* | S** | S** | S | S* | S+ | S+ | S** | S |
| CS-P3 | S+ | S* | S** | S** | S | S* | S+ | S+ | S** | S |
S = soluble, S+ = insoluble, S* = partially soluble and swelling, S** = partially soluble.
2.2.2. In Vitro Cytotoxicity Study
The cytotoxicity assay for the synthesized compounds was carried out based on MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. MTT assay is a colorimetric assay used for assessing cell viability and to measure cytotoxicity. As illustrated in Table 2, the results of the tested compounds CS-P1, CS-P2, and CS-P3 show a small variation between samples in comparison with the control. Several earlier research studies have demonstrated that CS and CSSB derivatives have little cellular toxicity. Consequently, CS has many applications in the medical field.36−38 The assay revealed that using a higher amount of the sample (200 mg) showed a cell viability of 89, 90, 90.1, and 91% for CS, CS-P1, CS-P2, and CS-P3, respectively. Conversely, a smaller amount (25 mg) of the tested compound showed a slight cytotoxicity of up to 2.5% compared with high concentrations, which is extremely suitable in medical applications. According to reported studies, compounds with higher than 75% cell viability are considered noncytotoxic.39 The cell viability assessment proved that the selection of these compound may depend on the antibacterial activity for their use in medical applications.
Table 2. Cytotoxicity Test of CS and Their SB Derivatives on the Viability of Mouse Fibroblast Cell Linesa.
| comp. conc. (mg) | viable cells in the presence of CS | viable cells in the presence of CS-P1 | viable cells in the presence of CS-P2 | viable cells in the presence of CS-P3 |
|---|---|---|---|---|
| 25 | 99 ± 0.83 | 99 ± 0.73 | 99 ± 0.60 | 99 ± 0.75 |
| 50 | 97 ± 0.73 | 99 ± 0.91 | 98.1 ± 1.3 | 99 ± 0.89 |
| 100 | 94 ± 0.63 | 98 ± 0.74 | 98 ± 1.2 | 98 ± 0.50 |
| 150 | 93 ± 0.88 | 97 ± 0.65 | 98 ± 0.62 | 96 ± 1.2 |
| 200 | 89 ± 0.53 | 90 ± 0.72 | 90.1 ± 0.74 | 91 ± 1.2 |
The experiment was repeated three times and the mean was calculated.
2.2.3. Antimicrobial Study
The inhibition zone technique was used to assess the antibacterial activity of the CSSB derivatives. The results are shown in Table 3. From the results, all of the CSSBs and CS have a similar impact on strains of both E. coli and K. pneumonia and is similar to that of Cs. The reported study demonstrated that CS could terminate the cell formation of S. aureus and E. coli.40 CSSBs showed antibacterial action against S. aureus with an inhibition zone of 22 ± 0.3, 20 ± 1.2, and 19 ± 0.62 mm for CS-P1, CS-P2, and CS-P3, respectively. The results also revealed that CS-P1, CS-P2, and CS-P3 have antibacterial action against S. mutans with an inhibition zone of 15 ± 0.89, 17 ± 0.50, and 18 ± 1.20 mm, respectively. Antifungal activity tests against two strains of fungi were carried out, and all verified CSSBs presented good results. The difference in inhibition between the two strains may result from the variances in the cell wall structures. From the result, it may be confirmed that the antibacterial action is through the breakage of the cell wall rather than the mechanism of interaction of CS derivatives with the DNA in the microorganism. The reaction of the active group on the CSSBs with the cell wall reduces permeability, which causes a shortage of substances in the cell, for example, amino acids, proteins, electrolytes, and lactate dehydrogenase. Therefore, the synthesized compounds lead to the inhibition of the metabolism of the bacteria and cause death.41
Table 3. Antimicrobial and Antifungal Activity Results of CS and CSSB Derivativesa.
| Gram-negative
bacteria |
Gram-positive
bacteria |
fungi |
||||
|---|---|---|---|---|---|---|
| comp. codes | E. coli | K. pneumonia | S. aureus | S. mutans | C. albicans | A. fumigatus |
| CS | 24 ± 0.63 | 26 ± 0.73 | NA | NA | 26 ± 0.79 | 16 ± 0.83 |
| CS-P1 | 22 ± 0.73 | 28 ± 0.91 | 22 ± 0.3 | 15 ± 0.89 | 34 ± 0.99 | 26 ± 0.91 |
| CS-P2 | 27 ± 0.83 | 27 ± 0.72 | 20 ± 1.2 | 17 ± 0.50 | 31 ± 1.29 | 25 ± 0.72 |
| CS-P3 | 22 ± 0.98 | 26 ± 0.65 | 19 ± 0.62 | 18 ± 1.20 | 26 ± 0.49 | 21 ± 0.65 |
NA means not detected.
Based on reported research,42 many mechanisms are proposed to clarify the action path of CS on microorganisms, which differs depending on the metabolic procedure and the structure of the cell wall. The first suggestion is the interruption of the cell wall of the organism because of the electrostatic attraction between the positively charged amine groups in CS and the negative residue group in the bacterial cell wall, such as −COO– or PO43–. The second mechanism suggested is the interaction of bacterial DNA with CS, which causes the inhibition of protein synthesis and mRNA by permeation of CS into the bacterial cell and then the nuclei. Another suggestion is based on the ability of CS to form a complex with metals, for instance, Zn2+, Mg2+, and Ca2+; these metals are important for bacterial metabolic processes and growth.
3. Conclusions
The search for new antibiotics has increased parallelly to the increase in the number of antibiotic-resistant microbes known as superbugs. CS might be a promising material in this field. CS with DD = 89% was extracted from the scales of Cyprinus carpio fish obtained from the local market. The structure of CS was characterized, and the DD % was determined by the acid–base titration method. Three novel CSSB derivatives with different parts as branches were synthesized, and their configurations were proved by FT-IR and 1H and 13C NMR spectroscopy. The new SB structure showed antibacterial activity against most microorganisms and fungi that were tested. The prepared CSSBs demonstrated, almost, no cytotoxic effect on mouse fibroblast cell lines. In light of the above findings, it is safe to say that the prepared CSSBs might be used in different biomedical fields with a high degree of safety and efficacy.
4. Materials and Methods
4.1. Materials
4.1.1. Chemicals and Raw Resources
Cyprinus carpio fish scales were collected from the local market in Kirkuk city, Iraq. Its DD was 89%, which was confirmed via a titration procedure.43 Ethanol (99.7%), hydrochloric acid (37%), 2-chloroquinoline-3-carbaldehyde, methyl orange, quinazoline-6-carbaldehyde, oxazole-4-carbaldehyde, and acetic acid were obtained from Merck (Germany). Sodium hydroxide pellets, sodium bicarbonate, hexamethylentetramine, magnesium sulfate, aqueous ammonia, sodium borohydride, tetrachloromethane, and trifluoroacetic were purchased from R&M Chemicals Pvt. Ltd., India. Aniline blue was purchased from Alpha Chemika, India. All reagents were of analytical grade and directly used without any further purification.
4.1.2. Devices
FT-IR spectra were obtained using a PerkinElmer System 2000 FT-IR spectrometer. A PerkinElmer TGA 7 thermogravimetric analyzer was applied. A PerkinElmer 2400 Series II analyzer was used to determine the percentage of N in the samples. A Bruker AC-400 NMR spectrometer was used to record the 1H and 13C NMR spectra. Deuterated dimethyl sulfoxide (d6-DMSO) was used as the solvent.
4.2. Microorganisms
Two eukaryote and four bacterial strains were used for assessing the antimicrobial effectiveness of CS and the newly prepared derivatives. The examined microorganisms included four species of bacteria, E. coli, K. pneumonia, S. aureus, and S. mutans, in addition to two species of fungi, C. albicans and A. fumigates. These organisms were collected from the biology department of Kirkuk University from patients who had a liver transplant. Nutrient agar was used as a culture medium. The strains were refreshed through inoculating Luria-Bertani (LB) culture medium,44,45 1% peptone, 0.5% yeast extract, and 1% NaCl and kept at 37 °C and pH 7 ±0.2 for 18 h. The inhibition ability of CS and SB derivatives was assessed. A 48-well plate was coated with samples, and 250 μL of the organism suspension (100 CFU/mL) was kept at 37 °C with LB culture. The latter was incubated at 37 °C for 1 day. Dimethyl sulfoxide (DMSO) was employed as a solvent and served as a control sample.
4.3. Cytotoxicity Evaluation
The cytotoxicity evaluation of CS and the new SB derivatives was carried out using MTT assay. A change in the reagent color to yellow was an indication of cell viability.40 Laminar flow cabinet biosafety class III was used to carry out all tests. A mouse fibroblast cell line (NIH3T3) was used in the test, which was grown in Dulbecco’s modified Eagle’s medium (DMEM), completed with trypsin/EDTA (100 μg/mL), and enriched with 10% FBS at 37 °C. Different amounts (25, 50, 100, 150, and 200 mg) of CS and the SB derivatives were used to assess their cytotoxicity. For the assessment of cytotoxicity of the newly prepared compounds, a microtiter plastic plate with 96 wells was coated with cells at a concentration of 10 × 103 cells per well and incubated overnight. The cells were incubated for 48 h with a Schiff base sample concentration of 90 μg/mL and alone as a negative control. At the end of the incubation time MTT (40 μL, 3 μg/mL) was added and kept at 37 °C for another 4 h. After the formation of crystals, sodium dodecyl sulfate (SDS) in distilled water (15%, 250 μL) was added to end the reaction. Doxorubicin (100 μg/mL) was used as a positive control under the same conditions. The absorbance was recorded at 595 and 620 nm as standard wavelengths. The tests were repeated three times (n = 3), and the mean value with mean ± SD was calculated. The SPSS 11 program was used to calculate the statistical significance between samples and the negative control. The below equation was used to calculate the percentage of change in cell viability46,47
| 3 |
4.4. Extraction of CS from Fish Scales
Approximately 100 g of fish scales was weighed and then washed and sun-dried for 3 days. The fish scales were crushed using a high-speed Hamilton beach blender. The scales were then immersed in hot ethanol (50–60 °C) for an hour to kill the germs and remove the pungent odor. The resulting sample (designated as C1) was washed with an ample amount of water and subjected to freeze-drying. C1 was treated with 5% HCl at 25 °C for 24 h to eliminate the CaCO3 component through a demineralization process. Then, 10.0 g of the sample was collected and labeled C2. The remaining sample was processed with a deproteinization step; 10% NaOH was used to remove the protein component at 60 °C. Then, 10.0 g of the sample was collected and labeled C3. The remaining sample was subjected to a deacetylation process by treating with NaOH (50%) under refluxing conditions to produce CS. Then, 10.0 g of the sample was collected and labeled C4.48
4.5. Synthesis of CSSB Derivatives
The new CSSB derivatives were synthesized according to a reported method.49 A gram of CS in 50 mL of glacial acetic acid (2%) was stirred at 25 °C for 7 h. A mixture of the aldehyde was dissolved in ethanol (1:1, molar ratio aldehyde to CS) that was added to the mixture gradually. The mixture was stirred and heated in a water bath at 50 °C for 10 h. Aqueous sodium hydroxide 6% was added to the reaction mixture until precipitation of the desired compound. The precipitate was collected and washed several times with distilled water and ethanol to remove any remaining materials. The products were filtered and dried in a vacuum oven at 60 °C overnight. The schematic diagram shown in Scheme 1 illustrates the synthesis routes to the new CSSBs.
4.6. Characterization of Extracted CS
4.6.1. Acid–Base Titration
4.6.1.1. Preparation of the Indicators
A solution of methyl orange and aniline blue (0.10 g of each, 100 mL of distilled water) was added to a beaker, separately. The mixture was then transferred into a volumetric flask (100 mL).
4.6.1.2. Titration Methods
In four clean and dry conical flasks, 0.30 g of each sample, C3, C4, CH, and CS, was added. Then, 30 mL of 0.1 M HC was added to each flask. Equivalent amounts of both methyl orange and aniline blue solutions were added to each set; the mixture was mixed with a glass rod until the color of the mixture became stable. The titration method was carried out for each sample set against NaOH (0.1 M) until the indicator color changed.
4.6.2. FT-IR Analysis
FT-IR spectra of CH, CS, C3, and C4 and the CSSB derivatives (CS-P1, CS-P2, and CS-P3) were recorded via a FT-IR spectroscope (Model 8400, Shimadzu). KBr was mixed with 2 mg of the sample and pressed to form a homogeneous disc with ≈0.5 mm thickness. The spectral region between 4000 and 400 cm–1 was scanned.
4.6.3. Thermogravimetric Investigation (TGA)
The thermal stability of deacetylated CS was evaluated; approximately 5 mg of CS was analyzed using a thermogravimetric analyzer (model 50/50H, Shimadzu). The heating was done as described from 60 to 750 °C at a rate of of 5 °C/min under 30 mL/min flow rate of nitrogen.50
Acknowledgments
M.O.M. wishes to thank the Ministry of Higher Education and Scientific Research, Iraq, for the award of a grant, and the authors acknowledge the Department of Biology, College of Science, Kirkuk University, Iraq, for their help with carrying out the biological tests.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01342.
General procedure and details of the treatment of CS and CH; tables show the results of the acid–base titration method and the characteristic absorption bands in the FT-IR spectra of standard and experimentally prepared CS; figures display the DD % of samples; TG and DTG plots; and 1H NMR, 13C NMR, and FT-IR spectra for all prepared compounds (PDF)
Author Contributions
All authors contributed to the writing of the manuscript. All authors have approved the final version of the manuscript. The photo in Figure 1 was taken by the authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Hamed A. A.; Abdelhamid I. A.; Saad G. R.; Elkady N. A.; Elsabee M. Z.. Synthesis, Characterization and Antimicrobial Activity of a Novel Chitosan Schiff Bases Based on Heterocyclic Moieties; Elsevier B.V., 2020; Vol. 153. [DOI] [PubMed] [Google Scholar]
- Muhd Julkapli N.; Akil H. M.; Ahmad Z. Preparation, Properties and Applications of Chitosan-Based Biocomposites/Blend Materials: A Review. Compos. Interfaces 2011, 18, 449–507. 10.1163/156855411X610232. [DOI] [Google Scholar]
- Casadidio C.; Peregrina D. V.; Gigliobianco M. R.; Deng S.; Censi R.; Di Martino P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. 10.3390/md17060369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matica M. A.; Aachmann F. L.; Tøndervik A.; Sletta H.; Ostafe V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. 10.3390/ijms20235889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Rodríguez R.; Espinosa-Andrews H.; Velasquillo-Martínez C.; García-Carvajal Z. Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: a review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. 10.1080/00914037.2019.1581780. [DOI] [Google Scholar]
- Molnár Á. The use of chitosan-based metal catalysts in organic transformations. Coord. Chem. Rev. 2019, 388, 126–171. 10.1016/j.ccr.2019.02.018. [DOI] [Google Scholar]
- Tang X.; Huang T.; Zhang S.; Wang W.; Zheng H. The role of sulfonated chitosan-based flocculant in the treatment of hematite wastewater containing heavy metals. Colloids Surf., A 2020, 585, 124070 10.1016/j.colsurfa.2019.124070. [DOI] [Google Scholar]
- Barbosa H. F. G.; Attjioui M.; Ferreira A. P. G.; Moerschbacher B. M.; Cavalheiro ÉT. G. New series of metal complexes by amphiphilic biopolymeric Schiff bases from modified chitosans: Preparation, characterization and effect of molecular weight on its biological applications. Int. J. Biol. Macromol. 2020, 145, 417–428. 10.1016/j.ijbiomac.2019.12.153. [DOI] [PubMed] [Google Scholar]
- Malekshah R. E.; Shakeri F.; Khaleghian A.; Salehi M. Developing a biopolymeric chitosan supported Schiff-base and Cu(II), Ni(II) and Zn(II) complexes and biological evaluation as pro-drug. Int. J. Biol. Macromol. 2020, 152, 846–861. 10.1016/j.ijbiomac.2020.02.245. [DOI] [PubMed] [Google Scholar]
- Antony R.; Arun T.; Manickam S. T. D. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019, 129, 615–633. 10.1016/j.ijbiomac.2019.02.047. [DOI] [PubMed] [Google Scholar]
- Sureshkumar B.; Mary Y. S.; Panicker C. Y.; Suma S.; Armaković S.; Armaković S. J.; Van Alsenoy C.; Narayana B. Quinoline derivatives as possible lead compounds for anti-malarial drugs: Spectroscopic, DFT and MD study. Arab. J. Chem. 2020, 13, 632–648. 10.1016/j.arabjc.2017.07.006. [DOI] [Google Scholar]
- Mishra M.; Agarwal S.; Dixit A.; Mishra V. K.; Kashaw V.; Agrawal R. K.; Kashaw S. K. Integrated computational investigation to develop molecular design of quinazoline scaffold as promising inhibitors of plasmodium lactate dehydrogenase. J. Mol. Struct. 2020, 1207, 127808 10.1016/j.molstruc.2020.127808. [DOI] [Google Scholar]
- Ibrahim T. S.; Bokhtia R. M.; AL-Mahmoudy A. M. M.; Taher E. S.; AlAwadh M. A.; Elagawany M.; Abdel-Aal E. H.; Panda S.; Gouda A. M.; Asfour H. Z.; Alhakamy N. A.; Youssif B. G. M. Design, synthesis and biological evaluation of novel 5-((substituted quinolin-3-yl/1-naphthyl) methylene)-3-substituted imidazolidin-2,4-dione as HIV-1 fusion inhibitors. Bioorg. Chem. 2020, 99, 103782 10.1016/j.bioorg.2020.103782. [DOI] [PubMed] [Google Scholar]
- Wang M.; Zhang G.; Wang Y.; Wang J.; Zhu M.; Cen S.; Wang Y. Design, synthesis and anti-influenza A virus activity of novel 2,4-disubstituted quinazoline derivatives. Bioorg. Med. Chem. Lett. 2020, 30, 127143 10.1016/j.bmcl.2020.127143. [DOI] [PubMed] [Google Scholar]
- Bouzian Y.; Karrouchi K.; Sert Y.; Lai C. H.; Mahi L.; Ahabchane N. H.; Talbaoui A.; Mague J. T.; Essassi E. M. Synthesis, spectroscopic characterization, crystal structure, DFT, molecular docking and in vitro antibacterial potential of novel quinoline derivatives. J. Mol. Struct. 2020, 1209, 127940 10.1016/j.molstruc.2020.127940. [DOI] [Google Scholar]
- Shao W. B.; Zheng Y. T.; Liu J. M.; Fu Y. H.; Qi P. Y.; Zhou X.; Wu Z. B.; Wang P. Y.; Yang S. Antibacterial activities against Ralstonia solanacearum and Xanthomonas oryzae pv. oryzae of 6-chloro-4-(4-substituted piperazinyl)quinazoline derivatives. Bioorg. Med. Chem. Lett. 2020, 30, 126912 10.1016/j.bmcl.2019.126912. [DOI] [PubMed] [Google Scholar]
- Huang Z.-H.; Yin L.-Q.; Guan L.-P.; Li Z.-H.; Tan C. Screening of Chalcone Analogs with Anti-depressant, Anti-inflammatory, Analgesic, and COX-2–inhibiting Effects. Bioorg. Med. Chem. Lett. 2020, 30, 127173 10.1016/j.bmcl.2020.127173. [DOI] [PubMed] [Google Scholar]
- Alafeefy A. M.; Kadi A. A.; Al-Deeb O. A.; El-Tahir K. E. H.; Al-Jaber N. A. Synthesis, analgesic and anti-inflammatory evaluation of some novel quinazoline derivatives. Eur. J. Med. Chem. 2010, 45, 4947–4952. 10.1016/j.ejmech.2010.07.067. [DOI] [PubMed] [Google Scholar]
- Das D.; Xie L.; Wang J.; Shi J.; Hong J. In vivo efficacy studies of novel quinazoline derivatives as irreversible dual EGFR/HER2 inhibitors, in lung cancer xenografts (NCI-H1975) mice models. Bioorg. Chem. 2020, 99, 103790 10.1016/j.bioorg.2020.103790. [DOI] [PubMed] [Google Scholar]
- Li B.; Zhu F.; He F.; Huang Q.; Liu X.; Wu T.; Zhao T.; Qiu Y.; Wu Z.; Xue Y.; Fang M. Synthesis and biological evaluations of N′-substituted methylene-4-(quinoline-4-amino) benzoylhydrazides as potential anti-hepatoma agents. Bioorg. Chem. 2020, 96, 103592 10.1016/j.bioorg.2020.103592. [DOI] [PubMed] [Google Scholar]
- Gao P.; Wang L.; Zhao L.; Zhang Q. Y.; Zeng K. W.; Zhao M. B.; Jiang Y.; Tu P. F.; Guo X. Y. Anti-inflammatory quinoline alkaloids from the root bark of Dictamnus dasycarpus. Phytochemistry 2020, 172, 112260 10.1016/j.phytochem.2020.112260. [DOI] [PubMed] [Google Scholar]
- Hejazi L.; Rezaee E.; Tabatabai S. A. Quinazoline-4(3H)-one derivatives as novel and potent inhibitors of soluble epoxide hydrolase: Design, synthesis and biological evaluation. Bioorg. Chem. 2020, 99, 103736 10.1016/j.bioorg.2020.103736. [DOI] [PubMed] [Google Scholar]
- Aguirre-Rentería S. A.; Carrizales-Castillo J. J. J.; del Rayo Camacho Corona M.; Hernández-Fernández E.; Garza-González E.; Rivas-Galindo V. M.; Arredondo-Espinoza E.; Avalos-Alanís F. G. Synthesis and in vitro evaluation of antimycobacterial and cytotoxic activity of new α,β-unsaturated amide, oxazoline and oxazole derivatives from L-serine. Bioorg. Med. Chem. Lett. 2020, 30, 127074 10.1016/j.bmcl.2020.127074. [DOI] [PubMed] [Google Scholar]
- Kenawy E. R.; Abdel-Hay F. I.; Tamer T. M.; Abo-Elghit Ibrahim E. M.; Mohy Eldin M. S. Antimicrobial activity of novel modified aminated chitosan with aromatic esters. Polym. Bull. 2020, 77, 1631–1647. 10.1007/s00289-019-02816-w. [DOI] [Google Scholar]
- Hemming E. B.; Masters A. F.; Perosa A.; Selva M.; Maschmeyer T. Single-Step Methylation of Chitosan Using Dimethyl Carbonate as a Green Methylating Agent. Molecules 2019, 24, 3986. 10.3390/molecules24213986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Fu C.; Wu S.; Liu G.; Guo J.; Su Z. Determination of the Deacetylation Degree of Chitooligosaccharides. Mar. Drugs 2017, 15, 332. 10.3390/md15110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowska-Biskup R.; Jarosińska D.; Rokita B.; Ulański P.; Rosiak J. M. Determination of degree of deacetylation of chitosan - Comparision of methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 17, 5–20. [Google Scholar]
- Brugnerotto J.; Lizardi J.; Goycoolea F. M.; Argüelles-Monal W.; Desbrières J.; Rinaudo M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. 10.1016/S0032-3861(00)00713-8. [DOI] [Google Scholar]
- Paldureţu C. C.; Apetroaei M. R.; Rǎu I.; Schroder V. Characterization of chitosan extracted from different romanian black sea crustaceans. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2018, 80, 13–24. [Google Scholar]
- Zhou H. Y.; Chen X. G.; Kong M.; Liu C. S.; Cha D. S.; Kennedy J. F. Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohydr. Polym. 2008, 73, 265–273. 10.1016/j.carbpol.2007.11.026. [DOI] [Google Scholar]
- Vasilev A.; Efimov M.; Bondarenko G.; Kozlov V.; Dzidziguri E.; Karpacheva G. Thermal behavior of chitosan as a carbon material precursor under IR radiation. IOP Conf. Ser.: Mater. Sci. Eng. 2019, 693, 012002 10.1088/1757-899X/693/1/012002. [DOI] [Google Scholar]
- Kolhe P.; Kannan R. M. Improvement in ductility of chitosan through blending and copolymerization with PEG: FTIR investigation of molecular interactions. Biomacromolecules 2003, 4, 173–180. 10.1021/bm025689+. [DOI] [PubMed] [Google Scholar]
- Tian F.; Liu Y.; Hu K.; Zhao B. Study of the depolymerization behavior of chitosan by hydrogen peroxide. Carbohydr. Polym. 2004, 57, 31–37. 10.1016/j.carbpol.2004.03.016. [DOI] [Google Scholar]
- Salama H. E.; Saad G. R.; Sabaa M. W. Synthesis, characterization and biological activity of Schiff bases based on chitosan and arylpyrazole moiety. Int. J. Biol. Macromol. 2015, 79, 996–1003. 10.1016/j.ijbiomac.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Synytsya A.; Kim W. J.; Kim S. M.; Pohl R.; Synytsya A.; Kvasnička F.; Čopíková J.; Park Y. I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. 10.1016/j.carbpol.2010.01.052. [DOI] [Google Scholar]
- Chen B.; Xing J.; Li M.; Liu Y.; Ji M. DOX@Ferumoxytol-Medical Chitosan as magnetic hydrogel therapeutic system for effective magnetic hyperthermia and chemotherapy in vitro. Colloids Surf., B 2020, 190, 110896 10.1016/j.colsurfb.2020.110896. [DOI] [PubMed] [Google Scholar]
- Benltoufa S.; Miled W.; Trad M.; Ben Slama R.; Fayala F. Chitosan hydrogel-coated cellulosic fabric for medical end-use: Antibacterial properties, basic mechanical and comfort properties. Carbohydr. Polym. 2020, 227, 115352 10.1016/j.carbpol.2019.115352. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Cao H.; Wang X. Synthesis and characterization of an injectable ε-polylysine/carboxymethyl chitosan hydrogel used in medical application. Mater. Chem. Phys. 2020, 248, 122902 10.1016/j.matchemphys.2020.122902. [DOI] [Google Scholar]
- Archana D.; Dutta J.; Dutta P. K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. 10.1016/j.ijbiomac.2013.03.002. [DOI] [PubMed] [Google Scholar]
- De Simone U.; Spinillo A.; Caloni F.; Avanzini M. A.; Coccini T. In vitro evaluation of magnetite nanoparticles in human mesenchymal stem cells: comparison of different cytotoxicity assays. Toxicol. Mech. Methods 2020, 30, 48–59. 10.1080/15376516.2019.1650151. [DOI] [PubMed] [Google Scholar]
- Kenawy E.; Abdel-hay F. I.; Eldin M. S. M.; Tamer T. M.; Abo-elghit E. M. Novel Aminated Chitosan-Aromatic Aldehydes Schiff Bases: Synthesis, Characterization and Bio-evaluation. Int. J. Adv. Res. 2015, 3, 563–572. [Google Scholar]
- Tamer T. M.; Hassan M. A.; Omer A. M.; Baset W. M. A.; Hassan M. E.; El-Shafeey M. E. A.; Eldin M. S. M. Synthesis, characterization and antimicrobial evaluation of two aromatic chitosan Schiff base derivatives. Process Biochem. 2016, 51, 1721–1730. 10.1016/j.procbio.2016.08.002. [DOI] [Google Scholar]
- Abdou E. S.; Nagy K. S. A.; Elsabee M. Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. 10.1016/j.biortech.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Jasso De Rodríguez D.; Hernández-Castillo D.; Rodríguez-García R.; Angulo-Sánchez J. L. Antifungal activity in vitro of Aloe vera pulp and liquid fraction against plant pathogenic fungi. Ind. Crops Prod. 2005, 21, 81–87. 10.1016/j.indcrop.2004.01.002. [DOI] [Google Scholar]
- Xiang Y.; Liu X.; Mao C.; Liu X.; Cui Z.; Yang X.; Yeung K. W. K.; Zheng Y.; Wu S. Infection-prevention on Ti implants by controlled drug release from folic acid/ZnO quantum dots sealed titania nanotubes. Mater. Sci. Eng. C 2018, 85, 214–224. 10.1016/j.msec.2017.12.034. [DOI] [PubMed] [Google Scholar]
- Bassyouni F. A.; Abu-Baker S. M.; Mahmoud K.; Moharam M.; El-Nakkady S. S.; Abdel-Rehim M. Synthesis and biological evaluation of some new triazolo[1,5-a]quinoline derivatives as anticancer and antimicrobial agents. RSC Adv. 2014, 4, 24131–24141. 10.1039/c3ra46961a. [DOI] [Google Scholar]
- Nada A.; Al-Moghazy M.; Soliman A. A. F.; Rashwan G. M. T.; Eldawy T. H. A.; Hassan A. A. E.; Sayed G. H. Pyrazole-based compounds in chitosan liposomal emulsion for antimicrobial cotton fabrics. Int. J. Biol. Macromol. 2018, 107, 585–594. 10.1016/j.ijbiomac.2017.09.031. [DOI] [PubMed] [Google Scholar]
- Islam M.; Masum S.; Rahman M. M.; Islam A.; Shaikh A. A. Preparation of Chitosan from Shrimp Shell and Investigation of Its Properties. Int. J. Basic Appl. Sci. 2011, 11, 77–80. [Google Scholar]
- Kenawy E.-R.; Ali S. S.; Al-Etewy M.; Sun J.; Wu J.; El-Zawawy N. Synthesis, characterization and biomedical applications of a novel Schiff base on methyl acrylate-functionalized chitosan bearing p-nitrobenzaldehyde groups. Int. J. Biol. Macromol. 2019, 122, 833–843. 10.1016/j.ijbiomac.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Neto C. G. T.; Giacometti J. A.; Job A. E.; Ferreira F. C.; Fonseca J. L. C.; Pereira M. R. Thermal analysis of chitosan based networks. Carbohydr. Polym. 2005, 62, 97–103. 10.1016/j.carbpol.2005.02.022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.