Figure 2.
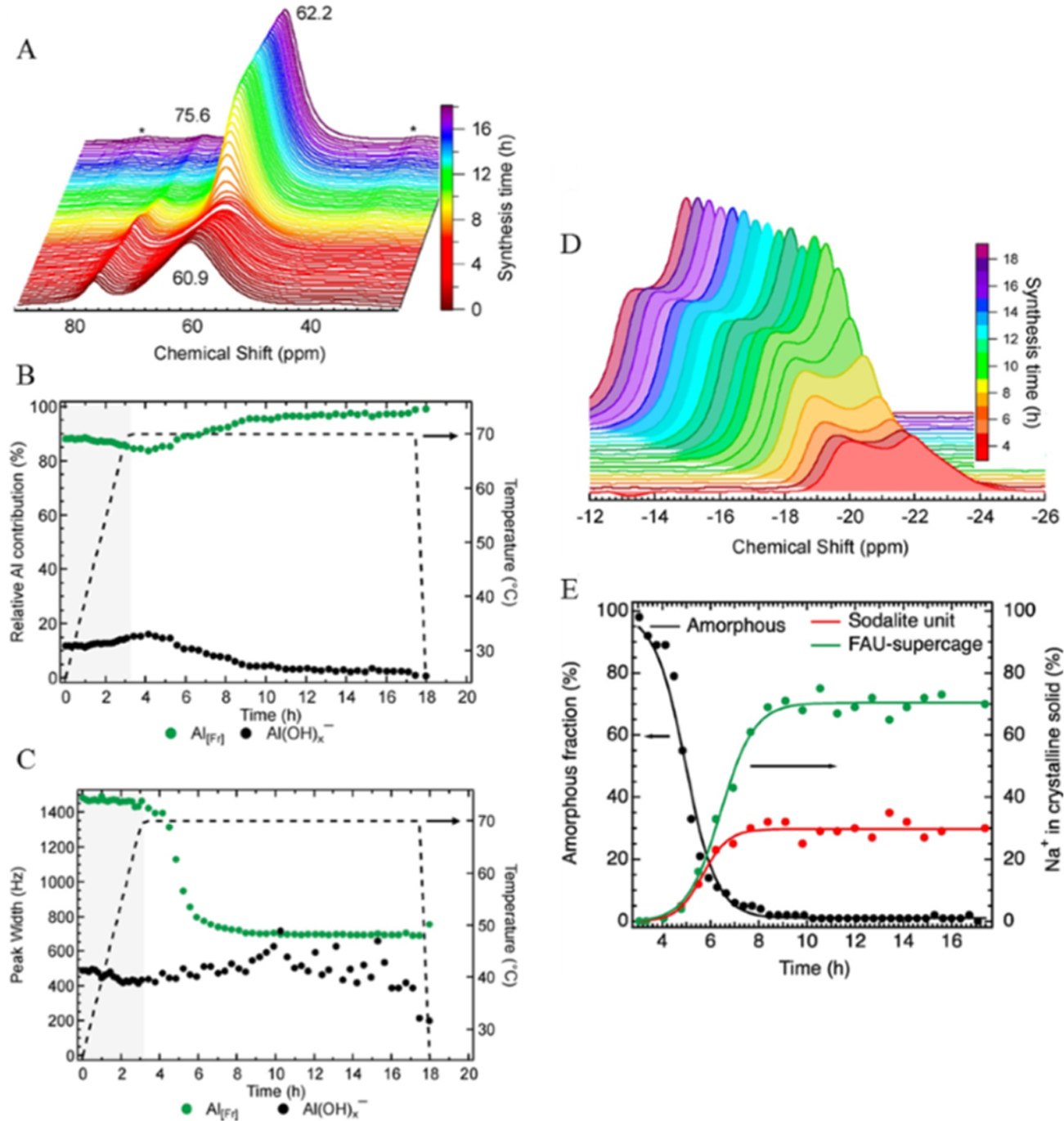
(A) In situ27Al MAS NMR spectra showing the changes during the synthesis of FAU. Deconvolution of the spectra (3 h heating period shaded in gray) led to modulation of the peak area (B) and line width (C) observed for liquid Al(OH)x− and solid tetrahedral Al (Al[Fr]). Changes in the spinning sideband associated with solid Na+ material as a function of synthesis time (D). A high-field peak and a low-field spinning sideband peak were identified at −22 and −20 ppm, respectively. (E) Kinetic transformation of amorphous material into crystalline FAU as directed by the speciation of Na+ ions [plotted as formed fraction of the final concentration of sodalite (−20 ppm) and the supercage (−22 ppm)] with lines included to guide the eyes. Reprinted with permission from ref 28. Copyright 2018 American Chemical Society.
