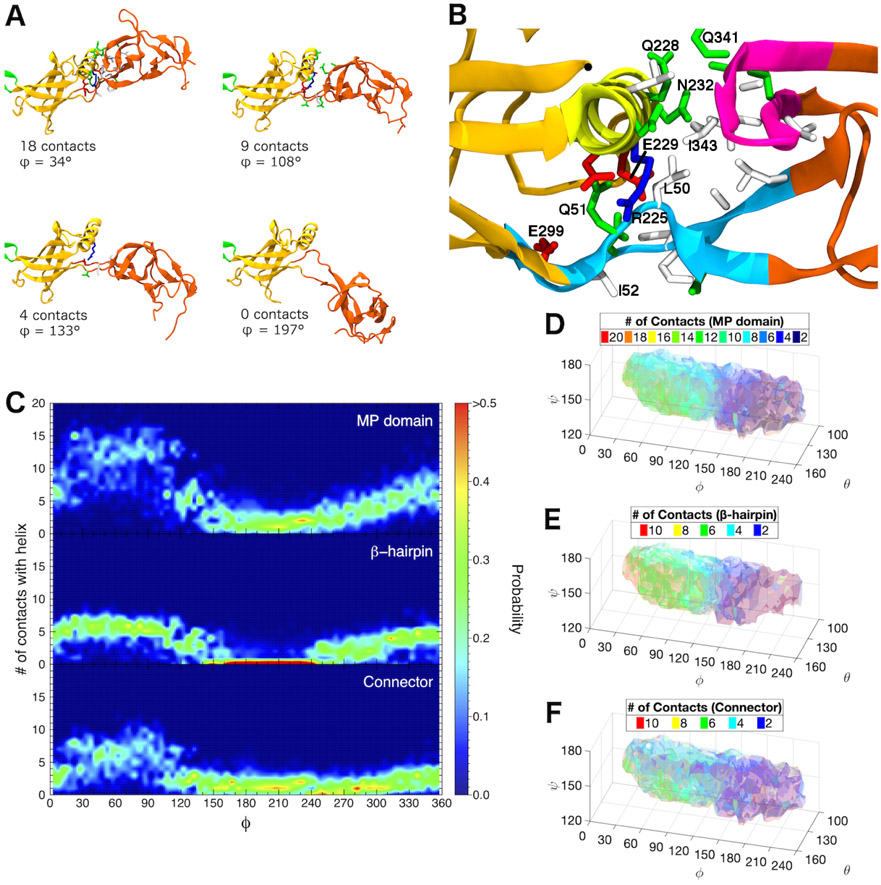
Figure 3:
Interactions between β-barrel and MP domains distinguish the two main basins of the conformational landscape of free AcrA monomers. (A) Snapshots from a single equilibrium simulation of free AcrA starting from the cis conformation. Protein backbone is shown in cartoon representation, with MP domain colored reddish-orange, β-barrel domain colored yellowish-orange, and lipoyl domain colored green. Contact residues on the MP domain and the α-helix of the β-barrel domain are shown in licorice representation with hydrogen atoms omitted, colored by residue type: (blue) positively charged, (red) negatively charged, (green) polar, and (white) hydrophobic. Two residues are in contact if their side chains are within 5 Å. (B) Close up of the interfacial site between the β-barrel domain and the MP domain. Helical residues 220 to 232 are colored yellow, MP β-hairpin residues 339 to 350 are colored magenta, and connector residues 48 to 53 and 299 to 307 are colored light blue. Important contact residues are labeled. (C) Distribution of the number of MP domain contacts with the β-barrel helix over the dihedral angle, ϕ, for (top) the entire MP domain, (center) the β-hairpin, and (bottom) the connector calculated from six 250-ns equilibrium simulations. (D)-(F) Number of MP domain contacts with the β-barrel helix calculated from our REUS simulations for (D) the entire MP domain, (E) the β-hairpin, and (F) the connector. For (D)-(F), isosurfaces are drawn in transparent colors, with their values labeled in the legends.