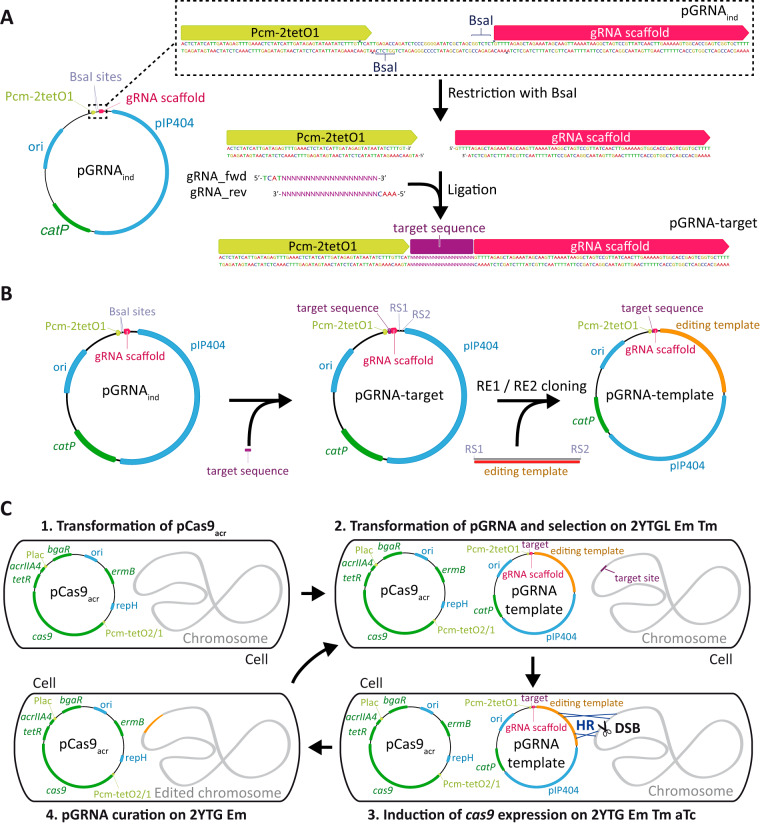
FIG 1.
Easy reprogramming of the two-plasmid CRISPR-Cas9 genetic tool for multiple genome editing. (A) Retargeting of the pGRNA plasmid by cloning two hybridized primers between the aTc-inducible promoter Pcm-2tetO1 and the gRNA scaffold. (B) Insertion of the editing template within the pGRNA plasmid by restriction enzyme-based cloning. (C) Iterative genome modification. Step 1, the strain is transformed with pCas9acr and transformants are selected on erythromycin-containing medium. Step 2, the resulting strain is transformed with a pGRNA editing plasmid, and transformants are selected on medium containing erythromycin, thiamphenicol, and lactose to activate the transcription of acrIIA4. Step 3, expression of cas9 is triggered on medium containing erythromycin and thiamphenicol and devoid of lactose so that transcription of acrIIA4 is no longer induced. The medium contains aTc to trigger the transcription of cas9 to select mutants in which the editing event occurred. Step 4, once aTc-resistant colonies have been confirmed, pGRNA is curated on medium containing erythromycin and no thiamphenicol, yielding cells ready for a new round of modification. ori, replication origin for E. coli; pIP404 and repH, compatible replication origins for C. acetobutylicum; catP, thiamphenicol-chloramphenicol resistance gene; ermB, erythromycin resistance gene; RS, restriction site; RE, restriction enzyme; DSB, double-strand break; HR, homologous recombination.