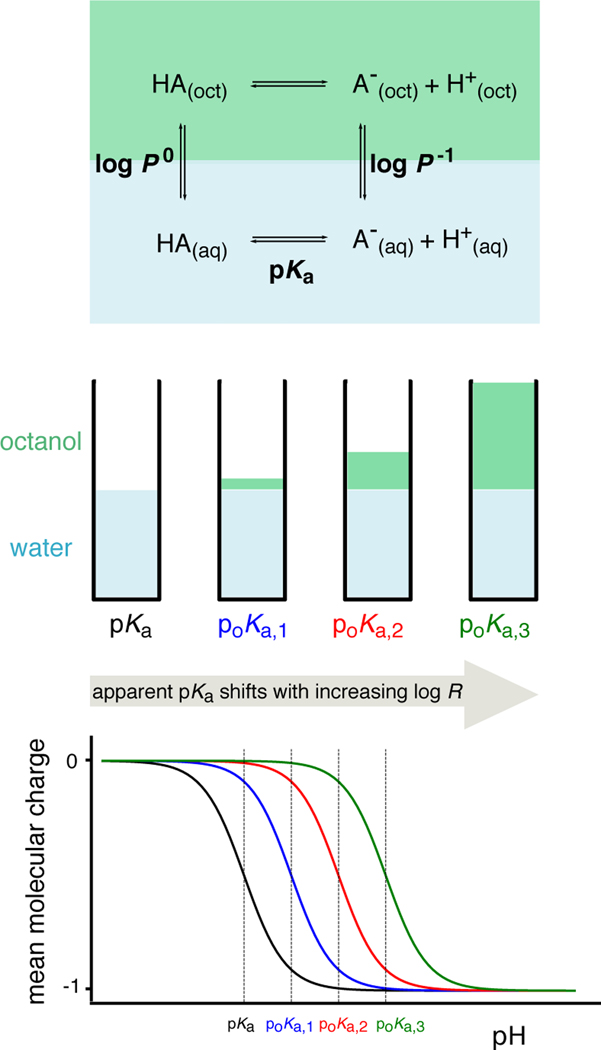
Figure 1. Potentiometric log P measurements are based on a model of ionization and partitioning equilibria [50].
Measurements of the pKa and apparent pKa (poKa) at three octanol-water volumetric ratios (log R) are performed to estimate the partition coefficients of neutral and ionized species, log P0 and log P−1, respectively. An ionization and partitioning equilibria model, along with estimated potentiometric titration curves, are shown for a monoprotic acid in this figure.