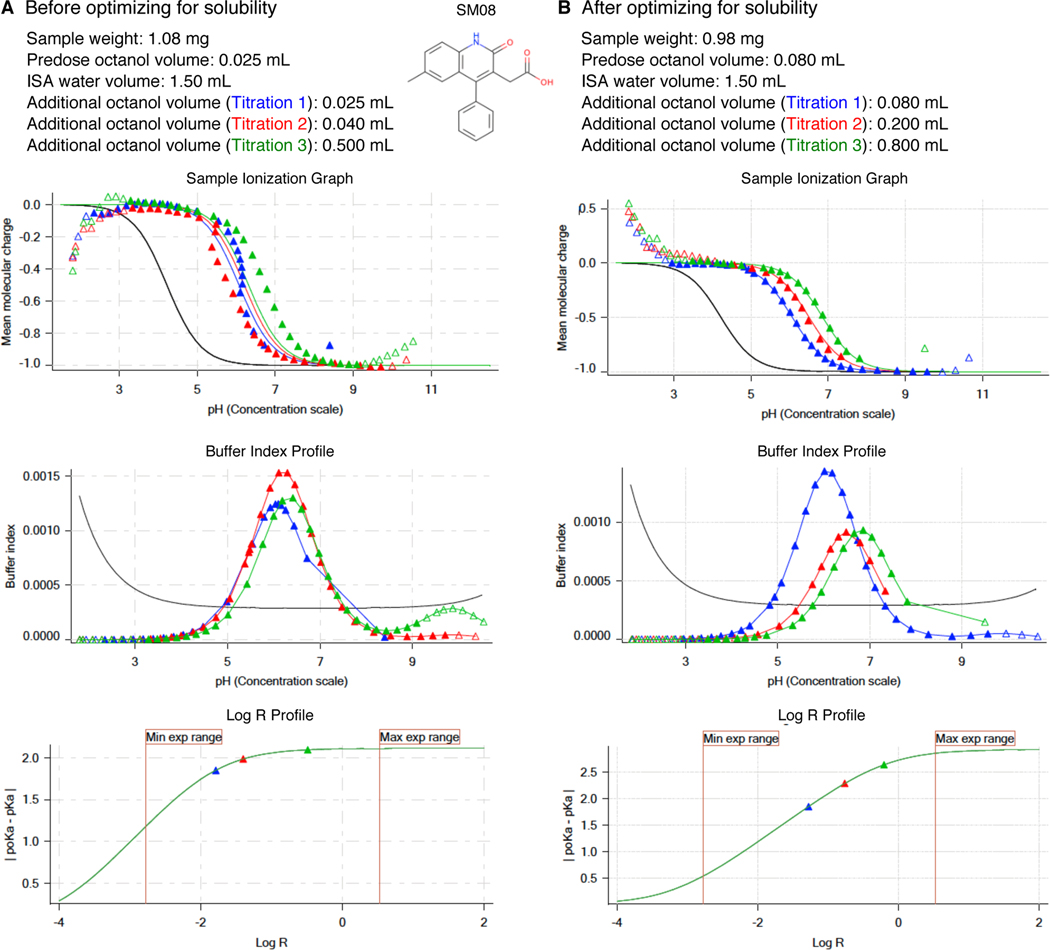
Figure 5. Potentiometric log P protocol optimization for SM08 to alleviate aqueous solubility problems.
Experimental results of initial protocol (A) and the optimized protocol (B) are shown for SM08. Both first (blue) and second (red) titrations in Sample Ionization Graph before optimization (A lower panel) show deviation from expected sigmoidal shape which is an indication of an insoluble analyte. This experiment with solubility issues led to log P and log P+1 estimates of 3.97 and 1.86. To eliminate precipitation, we could not lower analyte mass below 1 mg. Instead we were able to optimize the experimental protocol by increasing the predosed octanol volume and increasing additional octanol volumes added in each titration. Predosing octanol helps only with kinetic solubility issues. Larger octanol volumes can help to improve the experiment when thermodynamic solubility is the limitation, by allowing larger amounts of analyte partitioning into the octanol phase and reducing the analyte concentration in aqueous phase. Additional octanol volumes were selected such that they would also improve log R profile of the measurement. With optimized protocol (B) we achieved sample ionization profiles without any precipitation effects. log P and log P+1 were measured as 3.16 and 0.23. Once we achieved optimization of potentiometric log P protocol, triplicate measurements were collected using the same protocol.