Abstract
Objective
Adolescents with Type 1 diabetes (T1D) are vulnerable to diabetes-related distress and often struggle to complete self-management tasks needed to maintain blood glucose values in target range. One way that youth with T1D handle problems is through avoidant coping. The current study examined cross-time associations between avoidant coping style and diabetes outcomes and tested the possible mediating role of diabetes-related distress.
Method
Adolescents with T1D (N = 264) were assessed 4 times over 1 year to measure avoidant coping style, diabetes-related distress, adherence (on the basis of glucometer data and self-report), and glycemic control (hemoglobin A1c). Mediation and direct effects were tested across time using time-lagged autoregressive path models, making use of the repeated measurement of all constructs.
Results
The hypothesized mediation effect was found for all 3 diabetes outcomes. Higher levels of avoidant coping style were associated with greater diabetes-related distress at the subsequent time point, which was related in turn to fewer blood glucose checks, less frequent self-care behaviors, and poorer glycemic control (higher A1c) at the next assessment.
Conclusions
In the context of diabetes, an avoidant coping style may contribute to greater diabetes-specific distress followed by deterioration in self-management and glycemic control over time. Maladaptive coping styles are modifiable factors that offer an entry point into intervention before further difficulties can take hold.
Keywords: Type 1 diabetes, adolescents, diabetes distress, avoidant coping, longitudinal mediation
Management of Type 1 diabetes (T1D) is complex and demanding, like many other chronic health conditions. The specific management tasks for a person with T1D include close monitoring of blood glucose levels, dietary intake, and physical activity, as well as coordination of this information with insulin dosing and timing. Even with the most careful attention to these tasks, it is common to have blood glucose levels outside of the target range. Maximizing the time spent in target range is important for decreasing the risk of both acute and long-term complications (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, 2000; Diabetes Control and Complications Trial Research Group, 1993).
Adolescents with T1D struggle to keep up with the many demands of their daily diabetes regimen (Hood, Peterson, Rohan, & Drotar, 2009; Mortensen & Hougaard, 1997; Weissberg-Benchell et al., 1995). Recent data from the T1D Exchange clinic registry show that 84% of teens have out-of-range hemoglobin A1c (A1c) levels (Miller et al., 2015). Both physiologic and psychosocial factors contribute to these poor outcomes. While the physical changes of adolescent growth increase insulin resistance, the psychosocial changes experienced by adolescents often include less parental supervision (including oversight of T1D management) as they move toward independent activities and decision-making. They strive to fit in and appear “normal” with peers, most of whom are likely to not have T1D. As an added pressure, adolescents are evaluated on a quarterly basis by the medical team and are often prescribed increasingly intensive regimens to achieve target glucose levels. A better understanding of how adolescents cope with these interrelated developmental and diabetes-specific demands would lead to better prevention and intervention approaches to optimize time-in-target. The current study examines associations between one style of coping (avoidance) and later diabetes-related distress, adherence, and A1c.
Avoidant Coping Among Adolescents With T1D
How adolescents with T1D cope with problems, both in general and with diabetes specifically, is related to self-management behaviors and outcomes (Whittemore, Jaser, Guo, & Grey, 2010; Wysocki et al., 2008). Better adjustment and improved glycemic control are associated with primary control strategies, such as active problem solving and regulating one’s emotional responses, as well as secondary control strategies, such as thinking about a problem differently (Jaser & White, 2011). Contrary to these approaches, an avoidant coping style involves handling stressful situations by delaying attempts to solve a problem, disengaging from emotions and thoughts related to a problem, and otherwise downplaying the immediacy or seriousness of a problem. Although adolescents with T1D use a range of often adaptive coping strategies (Jaser et al., 2012), they appear to use more avoidant strategies than do younger children (Grey, Cameron, & Thurber, 1991; Hanson et al., 1989), and these coping processes have been associated with reduced adherence and higher A1c (Delamater, Kurtz, Bubb, White, & Santiago, 1987; Graue, Wentzel-Larsen, Bru, Hanestad, & Søvik, 2004; Hanson et al., 1989; Jaser & White, 2011; Reid, Dubow, Carey, & Dura, 1994; Seiffge-Krenke & Stemmler, 2003).
Most research on avoidant coping among adolescents with T1D has used a cross-sectional design, which is problematic for understanding the causal nature of these mechanisms. Does earlier avoidant coping affect later adjustment, adherence, and glycemic control? Or does an adolescent who is having more difficulties with diabetes naturally favor a more avoidant style of handling problems? Addressing these questions is important for theoretical development and the design of interventions. Some longitudinal research with this population has examined avoidant coping but has not made links between avoidant coping and later outcomes (Seiffge-Krenke & Stemmler, 2003), or it has focused on the explanatory effects of avoidant coping within a specific intervention (Jaser et al., 2014). There is a need not only to examine cross-time paths between avoidant coping and diabetes outcomes but also to assess possible explanatory mechanisms, or mediators, of this connection, such as diabetes-related distress.
Diabetes-Related Distress in Adolescence
Adolescents face multiple stressful aspects of diabetes care, which can include worries about not being perfect in management, fears about disappointing parents or doctors, reluctance to take time out of day-to-day activities for diabetes tasks, and embarrassment about standing out from their peers due to having a chronic health condition (Davidson, Penney, Muller, & Grey, 2004; Wysocki & Greco, 2006). These negative thoughts and feelings that accompany living with and managing diabetes are known as diabetes-related distress (Polonsky et al., 1995). One third of adolescents with T1D have significant diabetes-related distress, which is correlated with higher A1c and with reduced self-care behaviors (Hagger, Hendrieckx, Sturt, Skinner, & Speight, 2016; Hood et al., 2006; Weissberg-Benchell & Antisdel-Lomaglio, 2011). Despite the prevalence of this experience among youth, diabetes-related distress has been much less studied among adolescents compared to adults, and the mechanisms predicting this distress remain unknown. One untested possibility is that maladaptive coping fosters greater distress, which then impedes self-care behaviors and leads to worsening glycemic control. Evidence has suggested that greater avoidant coping leads to more distress over time for other populations of patients, including individuals with cancer and those undergoing burn treatment (Taylor & Stanton, 2007).
The Current Study
This study examined avoidant coping style, diabetes-related distress, adherence, and glycemic control in a cohort of adolescents with T1D who were assessed four times over a span of 1 year. There were two overarching aims. Aim 1 was to assess concurrent associations among avoidant coping style, diabetes-related distress, and diabetes outcomes. It was expected that avoidant coping and distress would be positively correlated and that both would be associated with poorer outcomes (reduced self-care, fewer blood glucose checks, and higher A1c). Aim 2 assessed diabetes-related distress as a mediator of the longitudinal association between avoidant coping and outcomes. We hypothesized that avoidant coping would lead over time to greater distress, which would in turn lead to reduced adherence and higher A1c values.
Method
Participants
The sample consisted of 264 adolescents with T1D who participated in the STePS study (Weissberg-Benchell, Rausch, Iturralde, Jedraszko, & Hood, 2016). Youth were recruited in two major U.S. cities using mailings, diabetes clinic flyers, and postings on hospital websites inviting youth to “learn strategies for managing diabetes.” Inclusion criteria were youth age (14–18), minimum diabetes duration of 1 year, total daily insulin of at least 0.5 units per 1 kg per day, and English fluency. Participants who were wards of the state, were on antidepressant medication, or had certain other diagnoses (major depression, chronic illness besides celiac or thyroid disease, developmental disorder, or other major mental disorder) were excluded.
Participants were randomized into one of two interventions—a resilience skills program or a dose-matched advanced diabetes education class—both conducted in nine group sessions every other week. The resilience skills intervention was based on a widely used adolescent depression prevention program, which was adapted to include content about T1D. Classes taught cognitive–behavioral concepts (e.g., understanding connections among thoughts, emotions, and behavior; challenging distorted thinking) and problem-solving skills (e.g., assertive communication, seeking social support). The advanced diabetes education group received lessons in, for example, nutrition, diabetes devices, management of glycemic fluctuations, and prevention of acute complications. Full details about the intervention study are published elsewhere (Weissberg-Benchell et al., 2016).
Table 1 shows baseline sample characteristics. Youth were ethnically diverse: 65.5% non-Hispanic White, 14.4% African American, 11.0% Hispanic, 2.3% Asian or Pacific Islander, 1.1% Native American or Alaska Native, and 5.7% classified as “Other.” Among those who enrolled, more were female (59.8%) than male. Mean diabetes duration was 6.88 years. About 70% administered insulin using a continuous subcutaneous insulin infusion pump versus multiple daily injections, which is comparable to the proportion in the T1D Exchange registry (Miller et al., 2015). Average A1c was 9.14%, which is higher than the 7.5% clinical guideline for youth (American Diabetes Association, 2015) but similar to that for other samples (Miller et al., 2015). More than 86% completed six or more intervention sessions. Between 87.9% and 90.9% completed follow-up Assessments 2 through 4. Youth did not differ on demographics, diabetes duration, insulin regimen, or A1c across intervention groups.
Table 1.
Sample Demographic and Baseline Characteristics
| Variable | n | % | M | SD |
|---|---|---|---|---|
| Age years | 15.7 | 1.09 | ||
| Diabetes duration years | 6.88 | 4.03 | ||
| Gender | ||||
| Male | 106 | 40.2 | ||
| Female | 158 | 59.8 | ||
| Race or ethnicity | ||||
| White, non-Hispanic | 173 | 65.5 | ||
| African American | 38 | 14.4 | ||
| Hispanic | 29 | 11.0 | ||
| Asian or Pacific Islander | 6 | 2.3 | ||
| Native American or Alaska Native | 3 | 1.1 | ||
| Reported as “Other” | 15 | 5.7 | ||
| Insulin regimen | ||||
| Multiple daily injections | 79 | 29.9 | ||
| Continuous subcutaneous insulin infusion | 185 | 70.1 | ||
| Group assignment | ||||
| Resilience | 133 | 50.4 | ||
| Education | 131 | 49.6 | ||
| Hemoglobin A1c | 9.14 | 1.92 |
Procedure
For the present study, participants completed assessments at four time points: at baseline and, after completing the intervention, at 4.5, 8, and 12 months postbaseline. To mitigate participant burden, we conducted assessments at convenient locations (e.g., clinic, youth’s home, public library). During visits, research staff obtained informed consent (from a guardian if the youth was a minor) and youth assent, administered self-report measures assessing broad and diabetes-specific adjustment, and took a small blood sample. Questionnaires were completed privately using a secure, Internet-based survey platform. Participants received graduated incentives ($35-$55) at each study visit. Study procedures were approved by the Institutional Review Boards of the two research sites.
Measures
Avoidant coping style
Participants completed the five-item Avoidance Style subscale of the Social Problem-Solving Inventory—Revised Short Form (SPSI-R:S; D’Zurilla, Nezu, & Maydeu-Olivares, 2002; baseline Cronbach’s alpha = .78). Items assess the tendency to evade or delay facing important life problems. Responses were made on a 5-point scale ranging from 0 (not at all true of me) to 4 (extremely true of me). The SPSI-R:S has been well validated with adolescent and medical populations (e.g., Hill-Briggs et al., 2006; Wade et al., 2012).
Diabetes-related distress
Past-month diabetes-related distress was assessed with the 26-item teen version of the Problem Areas in Diabetes Scale (PAID-T; Weissberg-Benchell & Antisdel-Lomaglio, 2011; baseline α = .95). The PAID–T asks respondents to rate how much a given problem area, such as “feeling overwhelmed by my diabetes regimen,” is bothering them on a 6-point scale ranging from 1 (not a problem) to 6 (serious problem).
Adherence
Blood glucose monitoring frequency was estimated via download of the previous 14-day history of blood glucose checks from participants’ glucometers; data were available from 89.4% to 95.3% of participants across the different assessments. Self-care behaviors were assessed using the 15-item Self Care Inventory (Weinger, Butler, Welch, & La Greca, 2005; baseline α = .77), which is a commonly used rating scale of adherence to diabetes self-care recommendations over the past 1 to 2 months. Items address issues such as “carry quick acting sugar to treat low blood glucose,” with responses rated on a 5-point scale ranging from 1 (never) to 5 (always).
A1c
Participants provided a capillary blood sample during assessment visits, and the sample was sent to the central laboratory for processing (Diabetes Diagnostic Laboratory at the University of Missouri; reference range = 4.0 to 6.0%).
Covariates
Variables with expected associations with diabetes outcomes—including age (in years), gender (0 = female, 1 = male), minority race or ethnicity (0 = White, 1 = Nonwhite), diabetes duration (in years), and baseline insulin regimen (0 = insulin pump, 1 = multiple daily injections)—were selected. Intervention group was also included (0 = education, 1 = resilience).
Analytic Plan
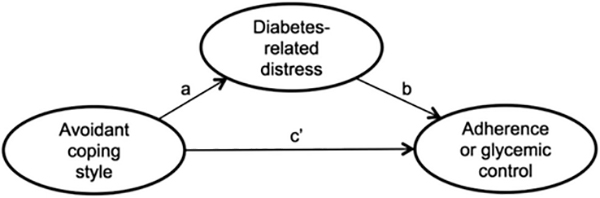
Aim 1, which was to examine concurrent associations among avoidant coping style, diabetes-related distress, and diabetes outcomes, was addressed through calculation of bivariate associations among predictor and outcome variables at each assessment point. To assess diabetes-related distress as a mediator of associations between avoidant coping style and diabetes outcomes (Aim 2), we used path modeling. Two types of associations were tested (see Figure 1): direct links between avoidant coping style and diabetes outcomes (path c’) and indirect links between avoidant coping style and the same outcomes via the hypothesized mediator diabetes-related distress (paths a and b). We selected a time-lagged autoregressive path modeling framework, which provides a strong test of longitudinal mediation by modeling interindividual changes along pathways using all measures at all time points, thus adjusting for earlier levels of each variable (Cole & Maxwell, 2003; Selig & Preacher, 2009). For mediation to exist, at minimum there must be a significant overall indirect effect; however, additional evaluation of the overall direct effect (while adjusting for the indirect effect) is helpful to characterize the nature of the mediation, whether as partial or full (Cole & Maxwell, 2003).
Figure 1.
Conceptual model of direct and indirect paths between avoidant coping style, diabetes-related distress, and diabetes outcomes. The indirect path is represented by a and b. The direct path is represented by c′.
To assess the overall indirect and direct effects, we combined calculations of all indirect or direct paths linking avoidant coping style with the given outcome using only time-lagged associations. For example, the overall indirect effect between avoidant coping style (ACS) and A1c via diabetes-related (DRD) combined the following indirect paths (subscripts represent assessment points):
The overall direct effect combined the following direct paths:
On the basis of recommendations by Shrout and Bolger (2002), these effects were evaluated by generating bias-corrected bootstrap confidence intervals (5,000 samples). Several possible models were tested and compared on the basis of fit with the data using conventional criteria for acceptability: comparative fit index (CFI) of .95 or greater and root-mean-square error of approximation (RMSEA) of less than .08 (Hu & Bentler, 1999). All models controlled for covariates. Path modeling was conducted in Mplus 7 using full-information maximum-likelihood estimation to handle missing data (Muthén & Muthen, 2012).
Results
Descriptive Statistics
Means, standard deviations, and correlations for study variables are shown in Table 2. Mean levels of each construct remained stable over the four time points, except for diabetes-related distress, which decreased, F(3, 618) = 11.18, p < .001, = .05. However, decreases in diabetes-related distress were equivalent across intervention groups (Group × Time), F(3, 618) = 1.33, p = .27, = .01. Neither intervention group saw changes in avoidant coping style overtime (Group × Time), F(3, 618) = .70, p = .55, = .003. On average, avoidant coping style items were reported by youth to be slightly true for themselves. Participants rated moderate levels of diabetes-related distress and self-care behaviors. Data on total blood glucose checks indicated that youth conducted an average of three to four daily finger checks over a 2-week period. Mean A1c was stable across the four assessments.
Table 2.
Descriptive Statistics for Study Variables at Each Assessment Point
| Pearson’s r |
||||||||
|---|---|---|---|---|---|---|---|---|
| Time point and variable | N | M | SD | ACS | DRD | BGC | SCB | A1c |
| Assessment 1 | ||||||||
| Avoidant coping style | 260 | 5.9 | 3.76 | — | ||||
| Diabetes-related distress | 260 | 73.1 | 26.7 | .36*** | — | |||
| Total blood glucose checks | 236 | 51.9 | 33.0 | −.18** | −.15* | — | ||
| Self-care behaviors | 260 | 54.1 | 7.85 | −.20** | −.46*** | .29*** | — | |
| A1c | 264 | 9.14 | 1.92 | .20** | .36*** | −.36*** | −.32*** | — |
| Assessment 2 | ||||||||
| Avoidant coping style | 229 | 6.25 | 3.92 | — | ||||
| Diabetes-related distress | 229 | 68.8 | 26.1 | .37*** | — | |||
| Total blood glucose checks | 221 | 49.1 | 31.3 | −.02 | −.16* | — | ||
| Self-care behaviors | 229 | 54.1 | 8.28 | −.26*** | −.56*** | .25*** | — | |
| A1c | 232 | 9.07 | 1.97 | .07 | .26*** | −.36*** | −.27*** | — |
| Assessment 3 | ||||||||
| Avoidant coping style | 237 | 5.85 | 3.91 | — | ||||
| Diabetes-related distress | 237 | 68.1 | 26.8 | .33*** | — | |||
| Total blood glucose checks | 219 | 49.5 | 29.6 | −.10 | −.14* | — | ||
| Self-care behaviors | 237 | 53.2 | 9.27 | −.31*** | −.53*** | .27*** | — | |
| A1c | 225 | 9.03 | 1.86 | .11 | .39*** | −.45*** | −.24*** | — |
| Assessment 4 | ||||||||
| Avoidant coping style | 240 | 6.03 | 4.43 | — | ||||
| Diabetes-related distress | 240 | 65.4 | 28.1 | .38*** | — | |||
| Total blood glucose checks | 228 | 48.8 | 31.1 | −.14* | −.15* | — | ||
| Self-care behaviors | 240 | 52.6 | 9.23 | −.29*** | −.55*** | .31*** | — | |
| A1c | 230 | 9.07 | 1.87 | .07 | .30*** | −.47*** | −.24*** | — |
Note. ACS = avoidant coping style; DRD = diabetes-related distress; BGC = blood glucose checks; SCB = self-care behaviors; A1c = hemoglobin A1c.
p < .05.
p < .01.
p < .001.
Differences were also assessed among study variables on the basis of covariates. Age was negatively related to self-care behaviors (r = −.18, p < .01). Youth with longer diabetes duration were older (r = .20, p < .01) and reported a less avoidant coping style (r = −.13, p < 05). Female participants reported more diabetes-related distress than did male, t(242.1) = 4.72, p < .001. Youth on insulin pumps versus injections had diabetes for 2.17 years longer, t(134.7) = 3.96, p < .001; 14.24 more blood glucose checks, t(234) = 3.03, p < .01; and a −.85% difference in A1c, t(262) = −3.36, p < .01, on average. Participants from a minority group had 11.91 fewer blood glucose checks, t(159.2) = −2.66, p < .01, and a +1.14% difference in A1c, t(262) = 4.66, p < .001, compared to White participants. There were no differences on any study variable or covariate across intervention groups. No other significant associations were found between covariates and study variables.
Aim 1: Associations Among Avoidant Coping Style, Diabetes-Related Distress, and Diabetes Outcomes
The overall pattern of associations was consistent with hypotheses. Avoidant coping style was positively related to diabetes-related distress at all assessments (rs = .33–.38, ps < .001). Avoidant coping style was consistently associated with reduced self-care behaviors (rs = −.31 to −.20, ps < .01). Negative associations with total blood glucose checks (rs = −.18 to −.02, ps = .006–.74) and positive associations with A1c (rs = .07–.20, ps = .001–.33) were significant at some but not all time points. Diabetes-related distress showed robust associations with total blood glucose checks (rs = −.14 to −.16, ps < .05), self-care behaviors (rs = −.46 to −.56, ps < .001), and A1c (rs = .26–.39, ps < .001).
Aim 2: Diabetes-Related Distress as a Mediator of Associations Between Avoidant Coping Style and Diabetes Outcomes
For all three diabetes outcomes, an initial model (Models 1a, 2a, and 3a, respectively) consisted of the following paths: avoidant coping style to diabetes-related distress at the next time point (path a), diabetes-related distress to diabetes outcome at the next time point (path b), and avoidant coping style to diabetes outcome at the next time point (path c′). Autoregressive paths were also estimated for each construct using one-time-point and two-time-point lags (stability paths). Concurrent associations among study variables were estimated within time point (residual covariance paths). For Models 1a, 2a, and 3a, all analogous paths were constrained to be equal (e.g., a paths were constrained to be equal to each other, b paths were constrained to be equal to each other, and so on). These initial models were compared to nested models with fewer constraints: Models 1b, 2b, and 3b allowed the a, b, and c′ paths to be freely estimated, and Models 1c, 2c, and 3c allowed the stability paths to be freely estimated. If freer models did not have superior fit, then this suggested that model paths were invariant across all time points in the study. Table 3 presents fit statistics for all of the tested models.
Table 3.
Initial and Nested Path Models With Fit Statistics for Each Diabetes Outcome
| Tested model | χ2 | df | CFI | RMSEA [90% CI] |
|---|---|---|---|---|
| Total blood glucose checks | ||||
| 1a. Initial constrained paths | 185.1 | 102 | .95 | .06 [.04, .07] |
| 1b. Freed a, b, and c′ paths | 181.0 | 96 | .94 | .06 [.05, .07] |
| 1c. Freed stability paths | 167.2 | 93 | .95 | .06 [.04, .07] |
| Self-care behaviors | ||||
| 2a. Initial constrained paths | 205.4 | 102 | .94 | .06 [.05, .07] |
| 2b. Freed a, b, and c′ paths | 199.8 | 96 | .94 | .06 [.05, .08] |
| 2c. Freed stability paths | 181.1 | 93 | .95 | .06 [.05, .07] |
| A1c | ||||
| 3a. Initial constrained paths | 192.9 | 102 | .96 | .06 [.05, .07] |
| 3b. Freed a, b, and c′ paths | 187.8 | 96 | .96 | .06 [.05, .07] |
| 3c. Freed stability paths | 174.6 | 93 | .96 | .06 [.04, .07] |
Note. Models 1a, 2a, and 3a are initial models used for comparison with subsequent models within the same outcome. CFI = comparative fit index; RMSEA = root-mean-square error of approximation; CI = confidence interval; A1c = hemoglobin A1c.
Initial models had acceptable fit; for example, Model 1a, χ2(102) = 185.1, CFI = .95, RMSEA = .06 (see statistics for all initial models in Table 3). Next, the models with freed constraints were tested. Because overall model fit indices did not improve and estimates for direct and indirect paths did not significantly change, the initial models were selected for reasons of parsimony. Parameter estimates and overall indirect and direct effects for the selected models are shown in Table 4. Due to the finding that the a, b, and c′ paths were invariant across assessments, a single estimate of these paths applies to all such paths regardless of time point.
Table 4.
Indirect, Direct, and Component Path Estimates for the Accepted Path Model for Each Diabetes Outcome
| Indirect effect ACS → DRD → diabetes outcome |
Direct effect ACS → diabetes outcome |
||||
|---|---|---|---|---|---|
| Tested model diabetes outcome | path a | path b | overall effect [95% CI] | path c′ | overall effect [95% CI] |
| Model 1a total blood glucose checks | .71*** | −.07* | −.08* [−.19, −.01] | −.01 | −.01 [−.34, .35] |
| Model 2a self-care behaviors | .72*** | −.04*** | −.05** [−.08, −.02] | −.21** | −.18** [−.29, −.08] |
| Model 3a A1c | .70*** | .003* | .004* [.001, .01] | .01 | .01 [−.01, .04] |
Note. Unstandardized path estimates are presented. Continuous variables were grand-mean-centered prior to analyses. ACS = avoidant coping style; DRD = diabetes-related distress; CI = confidence interval; A1c = hemoglobin A1c.
p < .05.
p < .01.
p < .001.
Avoidant coping style → diabetes-related distress → total blood glucose checks
The path model supported hypotheses. Both components of the indirect effect were significant (path a unstandardized estimate = .71, p < .001; b path unstandardized estimate = −.07, p < .05). The overall indirect effect was significant (unstandardized estimate = −.08; 95% bootstrap confidence interval [ − .19, − .01]). The overall direct effect was not significant after partialing out the indirect effect (unstandardized estimate = −.01; 95% bootstrap confidence interval [ − .34, .35]). Therefore, diabetes-related distress was a full mediator.
Avoidant coping style → diabetes-related distress → self-care behaviors
Consistent with hypotheses, both components of the indirect effect were significant (path a unstandardized estimate = .72, p < .001; path b unstandardized estimate = −.04, p < .001). The overall indirect effect was significant (unstandardized estimate = −.05, 95% bootstrap confidence interval [−.08, −.02]). The overall direct effect remained significant despite partialing out the indirect effect (unstandardized estimate = −.21, 95% bootstrap confidence interval [ − .29, −.08]). Therefore, the effect of avoidant coping style on self-care behaviors was partially mediated through diabetes-related distress but appears to be mediated through other variables not included in the model.
Avoidant coping style → diabetes-related distress → A1c
The path model supported hypotheses. The component paths of the indirect effect were significant (path a unstandardized estimate = .70, p < .001; path b unstandardized estimate = .003, p < .05; overall indirect effect unstandardized estimate = .004, 95% bootstrap confidence interval [.001, .01]). The overall direct effect was not significant (unstandardized estimate = .01, 95% bootstrap confidence interval [ − .01, .04]), indicating full mediation through diabetes-related distress.
Moderation by intervention assignment or age
Using the accepted models presented earlier, we performed moderation analyses to assess the role of intervention assignment (resilience vs. education) and baseline age (<16 vs. ≥16). This was achieved via two-group analyses whereby effects were constrained to be equal across groups and then freely estimated for each group. Because model fit did not significantly improve with the freed models, model effects were found to be equivalent regardless of intervention group and for both younger and older adolescents.
Alternative mediation via avoidant coping style
A final set of models explored the alternative possibility that avoidant coping style was also a mediator or that a bidirectional effect existed (i.e., avoidant coping style → diabetes-related distress and diabetes-related distress → avoidant coping style). While maintaining the indirect paths from Models 1a, 2a, and 3a, we added new paths to estimate indirect effects for diabetes-related distress → avoidant coping style → diabetes outcome. This approach simultaneously assessed mediation by avoidant coping style and by diabetes-related distress, as well as bidirectional effects. The resulting models fit well, χ2s(101) = 182.21–202.59, CFIs = .94–.96, RMSEAs = .06. The original indirect paths and overall indirect effects for avoidant coping style → diabetes-related distress → diabetes outcome were unchanged from the accepted models. The newly added indirect effects via avoidant coping style, however, were not significant (overall indirect effects = −.003 to <.001). There was also no significant bidirectional association, because diabetes-related distress did not predict later avoidant coping style (path a = .01, p = .11). Avoidant coping style predicted later self-care behaviors (path b = −.20, p < .01) but not later blood glucose checks (path b = −.01, p = .98) or A1c (path b = .01, p = .37). Thus, only diabetes-related distress was found to be a significant mediator, and results suggested that avoidant coping style led to later diabetes-related distress, rather than the opposite order of effects.
Discussion
Adolescents with T1D face many challenges in caring for this demanding condition. The present study focused on two psychological mechanisms, avoidant coping style and diabetes-related distress, which have been shown in past studies with adolescents to predict diabetes management and outcomes. Consistent with this past work, the current study found that a more avoidant coping style in general corresponds to greater levels of distress specific to diabetes and that each relates to reduced self-care behaviors, less frequent blood glucose monitoring, and poorer glycemic control, both concurrently and over time. Furthermore, diabetes-related distress acts as a mediator: Avoidant coping subsequently leads to more diabetes-related distress, which in turn leads to a worsened future diabetes outcome. In the case of blood glucose monitoring and glycemic control, the effect is fully explained by diabetes-related distress, whereas for self-reported adherence, there appear to be other variables in addition to diabetes-related distress that explain this link. To our knowledge, this is the only study that examines this longitudinal sequence of mechanisms.
Attempts to avoid facing problems through behavioral or emotional disengagement are one way for adolescents with diabetes to handle difficulties (Jaser & White, 2011). If an adolescent frequently avoids facing a diabetes problem, such as by delaying insulin treatment of high blood glucose readings, this may provide momentary relief of negative emotions associated with the situation. However, as suggested by the current findings, this pattern of avoidance may paradoxically lead to feelings that diabetes is overwhelming and unmanageable, and this diabetes-related distress may then interfere with further self-management. It is note-worthy that we found significant associations for avoidant coping that was general in nature rather than specific to diabetes problem solving. This link between general avoidance and T1D outcomes has been seen before with adolescents (Graue et al., 2004; Hanson et al., 1989; Seiffge-Krenke & Stemmler, 2003) and bears consideration for intervention development or refinement.
Some work with adolescents has suggested that coping skills training has positive impacts on adherence and glycemic control, although this approach does not target avoidant coping per se. A meta-analysis of T1D interventions with this age group (Hood, Rohan, Peterson, & Drotar, 2010) found that among various studies containing an adherence-promoting dimension, a multicomponent coping skills training program produced one of the stronger effects on glycemic control (Grey, Boland, Davidson, Li, & Tamborlane, 2000). The current study complements this work by highlighting the role of diabetes-related distress as a mechanism of change that appears to link coping with outcomes.
Another possible intervention approach could include addressing avoidant coping more directly. Avoidance processes are a central target of third-wave cognitive–behavioral treatments, especially acceptance and commitment therapy (ACT; Hayes, Luoma, Bond, Masuda, & Lillis, 2006). There is initial evidence of efficacy for ACT with adolescent medical and nonmedical populations (Swain, Hancock, Dixon, & Bowman, 2015; Wicksell, Kanstrup, Kemani, Holmstrom, & Olsson, 2015), but the development of T1D-specific ACT interventions with adolescents remains preliminary (Hadlandsmyth, White, Nesin, & Greco, 2013). It is possible that embedding general coping work with situations that generate diabetes-related distress may offer a more robust intervention to achieve more time-in-target goals.
The present study also raises the question of what coping skills would be more adaptive than avoidance. Past mediation analyses have found that increases in primary control coping (via strategies that focus on the problem and related emotions) and secondary control coping (applying adaptive thinking strategies) explained benefits to quality of life among adolescents with T1D participating in a coping skills training program (Jaser et al., 2014). Further analyses of the effects of adaptive coping on diabetes-related distress, adherence, and glycemic control would be beneficial.
Avoidant coping may be an early sign of future difficulties with diabetes-related distress and T1D outcomes and could be targeted for assessment and intervention during diabetes care visits. There has been an increased call to screen for depression and other patient-reported outcomes during pediatric visits (Huang, Revicki, & Schwartz, 2014; Siu, 2016) due to their demonstrated associations with health outcomes, including for adolescents with T1D (Hilliard, Herzer, Dolan, & Hood, 2011). Yet, it should be noted that screening procedures for depression are relatively well defined (Corathers et al., 2013), whereas there is little understanding of how to screen for adolescents’ problematic coping behaviors. It would be worthwhile to investigate practical ways to distinguish clinically significant avoidance among patients because these strategies appear to be modifiable and related not only to psychological distress but also to regimen adherence and glycemic control.
The current study has some important limitations. Given that the sample was part of a clinical trial and that participants were enrolled in one of two intervention groups, it is possible that intervention participation influenced the mediation effects, perhaps by causing the observed decrease in diabetes-related distress. This possibility was partially addressed through statistical techniques, which found no differences between intervention groups in study variable means, in changes in diabetes-related distress or avoidant coping style, or in the overall mediation model. Nor were mediation effects altered by timing; indirect paths were equivalent regardless of whether measures were collected during or after the intervention phase. Avoidant coping or distress may have been mitigated by both interventions through distinct mechanisms (e.g., metacognitive skill building in the resilience group and improved diabetes knowledge in the education group), and perhaps these changes affected our findings. Past research has demonstrated the benefits of both cognitive-behavioral therapy and psychoeducation in addressing adolescents’ diabetes-related distress (Murphy, Rayman, & Skinner, 2006; Serlachius et al., 2016). Aside from this issue of intervention effects, limitations are also posed by possible selection biases. The requirement of adolescents to participate in group intervention sessions and the exclusion of those with major depression may have resulted in a sample with lower than typical levels of avoidant coping behavior and diabetes-related distress. Replication of the current findings with a nonintervention study would test the relevance of these mediation processes for a broader range of adolescents. It would also be valuable to assess the roles of avoidant coping style and diabetes-related distress over a longer period of time, especially as individuals face the opportunities and challenges of greater autonomy in young adulthood. Age was not related to coping or distress, nor did it moderate mediation effects, but this may have been due to a narrow time frame and age range, which could be expanded in future studies.
Strengths of this study include the use of repeated measurement of psychological and diabetes health processes at four assessment points with a large, multiethnic sample from two geographic areas in the United States. To our knowledge, this is the first study to examine indirect effects of avoidant coping style via diabetes-related distress on diabetes health outcomes. An additional strength was that this study replicated the mediation effect with three different diabetes outcomes using three distinct measurement methods: youth-reported self-care behaviors, blood glucose monitoring based on glucometer data, and A1c assessed from a blood draw.
Conclusions
It is important to understand factors contributing to diabetes-related distress among adolescents with T1D to inform treatment with this at-risk population. Avoidant coping, which ostensibly seeks to reduce the discomfort associated with facing day-to-day problems, is one of many strategies used by adolescents. The current study demonstrates that, in the context of diabetes, an avoidant coping style may contribute to greater diabetes-specific distress followed by deterioration in self-management and glycemic control over time. Maladaptive coping styles are modifiable factors that offer an entry point into intervention before further adjustment or health difficulties can take hold.
Acknowledgments
We are grateful to the adolescents who generously participated in the study. We also thank the following staff members who worked tirelessly to enroll and retain participants: Sarah Hanes, Aneta Jedraszko, Paige Staudenmaier, and Sarah Woods. This research was supported by (National Institutes of Health) NIH R01 DK 090030 (Jill Weissberg-Benchell, Korey K. Hood, PIs).
Contributor Information
Esti Iturralde, Stanford University School of Medicine.
Jill Weissberg-Benchell, Northwestern University Feinberg School of Medicine/Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, Illinois.
Korey K. Hood, Stanford University School of Medicine
References
- American Diabetes Association. (2015). 6. Glycemic targets. Diabetes Care, 38(Suppl.), S33–S40. 10.2337/dc15-S009 [DOI] [PubMed] [Google Scholar]
- Cole DA, & Maxwell SE (2003). Testing mediational models with longitudinal data: Questions and tips in the use of structural equation modeling. Journal of Abnormal Psychology, 112, 558–577. 10.1037/0021-843X.112.4.558 [DOI] [PubMed] [Google Scholar]
- Corathers SD, Kichler J, Jones NH, Houchen A, Jolly M, Morwessel N, … Hood KK (2013). Improving depression screening for adolescents with type 1 diabetes. Pediatrics, 132(5), e1395–e1402. 10.1542/peds.2013-0681 [DOI] [PubMed] [Google Scholar]
- Davidson M, Penney ED, Muller B, & Grey M (2004). Stressors and self-care challenges faced by adolescents living with type 1 diabetes. Applied Nursing Research, 17, 72–80. 10.1016/j.apnr.2004.02.006 [DOI] [PubMed] [Google Scholar]
- Delamater AM, Kurtz SM, Bubb J, White NH, & Santiago JV (1987). Stress and coping in relation to metabolic control of adolescents with type 1 diabetes. Journal of Developmental and Behavioral Pediatrics, 8, 136–140. 10.1097/00004703-198706000-00002 [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. (2000). Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. New England Journal of Medicine, 342, 381–389. 10.1056/NEJM200002103420603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group. (1993). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine, 329, 977–986. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- D’Zurilla TJ, Nezu AM, & Maydeu-Olivares A (2002). Social Problem-Solving Inventory—Revised: Technical manual. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Graue M, Wentzel-Larsen T, Bru E, Hanestad BR, & Søvik O (2004). The coping styles of adolescents with type 1 diabetes are associated with degree of metabolic control. Diabetes Care, 27, 1313–1317. 10.2337/diacare.27.6.1313 [DOI] [PubMed] [Google Scholar]
- Grey M, Boland EA, Davidson M, Li J, & Tamborlane WV (2000). Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. Journal of Pediatrics, 137, 107–113. 10.1067/mpd.2000.106568 [DOI] [PubMed] [Google Scholar]
- Grey M, Cameron ME, & Thurber FW (1991). Coping and adaptation in children with diabetes. Nursing Research, 40, 144–149. 10.1097/00006199-199105000-00004 [DOI] [PubMed] [Google Scholar]
- Hadlandsmyth K, White KS, Nesin AE, & Greco LA (2013). Proposing an acceptance and commitment therapy intervention to promote improved diabetes management in adolescents: A treatment conceptualization. International Journal of Behavioral Consultation and Therapy, 7, 12–15. 10.1037/h0100960 [DOI] [Google Scholar]
- Hagger V, Hendrieckx C, Sturt J, Skinner TC, & Speight J (2016). Diabetes distress among adolescents with type 1 diabetes: A systematic review. Current Diabetes Reports, 16: 9 10.1007/s11892-015-0694-2 [DOI] [PubMed] [Google Scholar]
- Hanson CL, Cigrang JA, Harris MA, Carle DL, Relyea G, & Burghen GA (1989). Coping styles in youths with insulin-dependent diabetes mellitus. Journal of Consulting and Clinical Psychology, 57, 644–651. 10.1037/0022-006X.57.5.644 [DOI] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, & Lillis J (2006). Acceptance and commitment therapy: Model, processes and outcomes. Behaviour Research and Therapy, 44, 1–25. 10.1016/j.brat.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Hill-Briggs F, Gary TL, Yeh H-C, Batts-Turner M, Powe NR, Saudek CD, & Brancati FL (2006). Association of social problem solving with glycemic control in a sample of urban African Americans with type 2 diabetes. Journal of Behavioral Medicine, 29, 69–78. 10.1007/s10865-005-9037-0 [DOI] [PubMed] [Google Scholar]
- Hilliard ME, Herzer M, Dolan LM, & Hood KK (2011). Psychological screening in adolescents with type 1 diabetes predicts outcomes one year later. Diabetes Research and Clinical Practice, 94, 39–44. 10.1016/j.diabres.2011.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood KK, Huestis S, Maher A, Butler D, Volkening L, & Laffel LMB (2006). Depressive symptoms in children and adolescents with type 1 diabetes. Diabetes Care, 29, 1389–1391. 10.2337/dc06-0087 [DOI] [PubMed] [Google Scholar]
- Hood KK, Peterson CM, Rohan JM, & Drotar D (2009). Association between adherence and glycemic control in pediatric type 1 diabetes: A meta-analysis. Pediatrics, 124(6), e1171–e1179. 10.1542/peds.2009-0207 [DOI] [PubMed] [Google Scholar]
- Hood KK, Rohan JM, Peterson CM, & Drotar D (2010). Interventions with adherence-promoting components in pediatric type 1 diabetes: Meta-analysis of their impact on glycemic control. Diabetes Care, 33, 1658–1664. 10.2337/dc09-2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Huang IC, Revicki DA, & Schwartz CE (2014). Measuring pediatric patient-reported outcomes: Good progress but a long way to go. Quality of Life Research, 23, 747–750. 10.1007/s11136-013-0607-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser SS, Faulkner MS, Whittemore R, Jeon S, Murphy K, Delamater A, & Grey M (2012). Coping, self-management, and adaptation in adolescents with type 1 diabetes. Annals of Behavioral Medicine, 43, 311–319. 10.1007/s12160-012-9343-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser SS, & White LE (2011). Coping and resilience in adolescents with type 1 diabetes. Child: Care, Health and Development, 37, 335–342. 10.1111/j.1365-2214.2010.01184.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser SS, Whittemore R, Chao A, Jeon S, Faulkner MS, & Grey M (2014). Mediators of 12-month outcomes of two Internet interventions for youth with type 1 diabetes. Journal of Pediatric Psychology, 39, 306–315. 10.1093/jpepsy/jst081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, … Tamborlane, W. V. (2015). Current state of type 1 diabetes treatment in the U. S.: Updated data from the T1D Exchange clinic registry. Diabetes Care, 38, 971–978. 10.2337/dc15-0078 [DOI] [PubMed] [Google Scholar]
- Mortensen HB, & Hougaard P (1997). Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. Diabetes Care, 20, 714–720. 10.2337/diacare.20.5.714 [DOI] [PubMed] [Google Scholar]
- Murphy HR, Rayman G, & Skinner TC (2006). Psycho-educational interventions for children and young people with type 1 diabetes. Diabetic Medicine, 23, 935–943. 10.1111/j.1464-5491.2006.01816.x [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2012). Mplus users guide (7th ed.). Los Angeles, CA: Author. [Google Scholar]
- Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, & Schwartz CE (1995). Assessment of diabetes-related distress. Diabetes Care, 18, 754–760. 10.2337/diacare.18.6.754 [DOI] [PubMed] [Google Scholar]
- Reid GJ, Dubow EF, Carey TC, & Dura JR (1994). Contribution of coping to medical adjustment and treatment responsibility among children and adolescents with diabetes. Journal of Developmental and Behavioral Pediatrics, 15, 327–335. 10.1097/00004703-199410000-00003 [DOI] [PubMed] [Google Scholar]
- Seiffge-Krenke I, & Stemmler M (2003). Coping with everyday stress and links to medical and psychosocial adaptation in diabetic adolescents. Journal of Adolescent Health, 33, 180–188. 10.1016/S1054-139X(02)00707–3 [DOI] [PubMed] [Google Scholar]
- Selig JP, & Preacher KJ (2009). Mediation models for longitudinal data in developmental research. Research in Human Development, 6, 144–164. 10.1080/15427600902911247 [DOI] [Google Scholar]
- Serlachius AS, Scratch SE, Northam EA, Frydenberg E, Lee KJ, & Cameron FJ (2016). A randomized controlled trial of cognitive behaviour therapy to improve glycaemic control and psychosocial well-being in adolescents with type 1 diabetes. Journal of Health Psychology, 21, 1157–1169. 10.1177/1359105314547940 [DOI] [PubMed] [Google Scholar]
- Shrout PE, & Bolger N (2002). Mediation in experimental and non-experimental studies: New procedures and recommendations. Psychological Methods, 7, 422–445. 10.1037/1082-989X.7.4.422 [DOI] [PubMed] [Google Scholar]
- Siu AL (2016). Screening for depression in children and adolescents: Us preventive services task force recommendation statement. Pediatrics, 137(3): e20154467 10.1542/peds.2015-4467 [DOI] [PubMed] [Google Scholar]
- Swain J, Hancock K, Dixon A, & Bowman J (2015). Acceptance and commitment therapy for children: A systematic review of intervention studies. Journal of Contextual Behavioral Science, 4, 73–85. 10.1016/j.jcbs.2015.02.001 [DOI] [Google Scholar]
- Taylor SE, & Stanton AL (2007). Coping resources, coping processes, and mental health. Annual Review of Clinical Psychology, 3, 377–401. 10.1146/annurev.clinpsy.3.022806.091520 [DOI] [PubMed] [Google Scholar]
- Wade SL, Walz NC, Carey J, McMullen KM, Cass J, Mark E, & Yeates KO (2012). A randomized trial of teen online problem solving: Efficacy in improving caregiver outcomes after brain injury. Health Psychology, 31, 767–776. 10.1037/a0028440 [DOI] [PubMed] [Google Scholar]
- Weinger K, Butler HA, Welch GW, & La Greca AM (2005). Measuring diabetes self-care: A psychometric analysis of the Self-Care Inventory-Revised with adults. Diabetes Care, 28, 1346–1352. 10.2337/diacare.28.6.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg-Benchell J, & Antisdel-Lomaglio J (2011). Diabetes-specific emotional distress among adolescents: Feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatric Diabetes, 12, 341–344. http://dx.doi.Org/10.1111/j.1399-5448.2010.00720.x [DOI] [PubMed] [Google Scholar]
- Weissberg-Benchell J, Glasgow AM, Tynan WD, Wirtz P, Turek J, & Ward J (1995). Adolescent diabetes management and mismanagement. Diabetes Care, 18, 77–82. 10.2337/diacare.18.1.77 [DOI] [PubMed] [Google Scholar]
- Weissberg-Benchell J, Rausch J, Iturralde E, Jedraszko A, & Hood K (2016). A randomized clinical trial aimed at preventing poor psychosocial and glycemic outcomes in teens with type 1 diabetes (T1D). Contemporary Clinical Trials, 49, 78–84. 10.1016/j.cct.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore R, Jaser S, Guo J, & Grey M (2010). A conceptual model of childhood adaptation to type 1 diabetes. Nursing Outlook, 58, 242–251. 10.1016/j.outlook.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicksell RK, Kanstrup M, Kemani MK, Holmstrom L, & Olsson GL (2015). Acceptance and commitment therapy for children and adolescents with physical health concerns. Current Opinion in Psychology, 2, 1–5. 10.1016/j.copsyc.2014.12.029 [DOI] [Google Scholar]
- Wysocki T, & Greco P (2006). Social support and diabetes management in childhood and adolescence: Influence of parents and friends. Current Diabetes Reports, 6, 117–122. 10.1007/s11892-006-0022-y [DOI] [PubMed] [Google Scholar]
- Wysocki T, Iannotti R, Weissberg-Benchell J, Laffel L, Hood K, Anderson B, & Chen R (2008). Diabetes problem solving by youths with type 1 diabetes and their caregivers: Measurement, validation, and longitudinal associations with glycemic control. Journal of Pediatric Psychology, 33, 875–884. 10.1093/jpepsy/jsn024 [DOI] [PMC free article] [PubMed] [Google Scholar]