Figure 1. Symmetric and asymmetric cleavage of β-carotene.
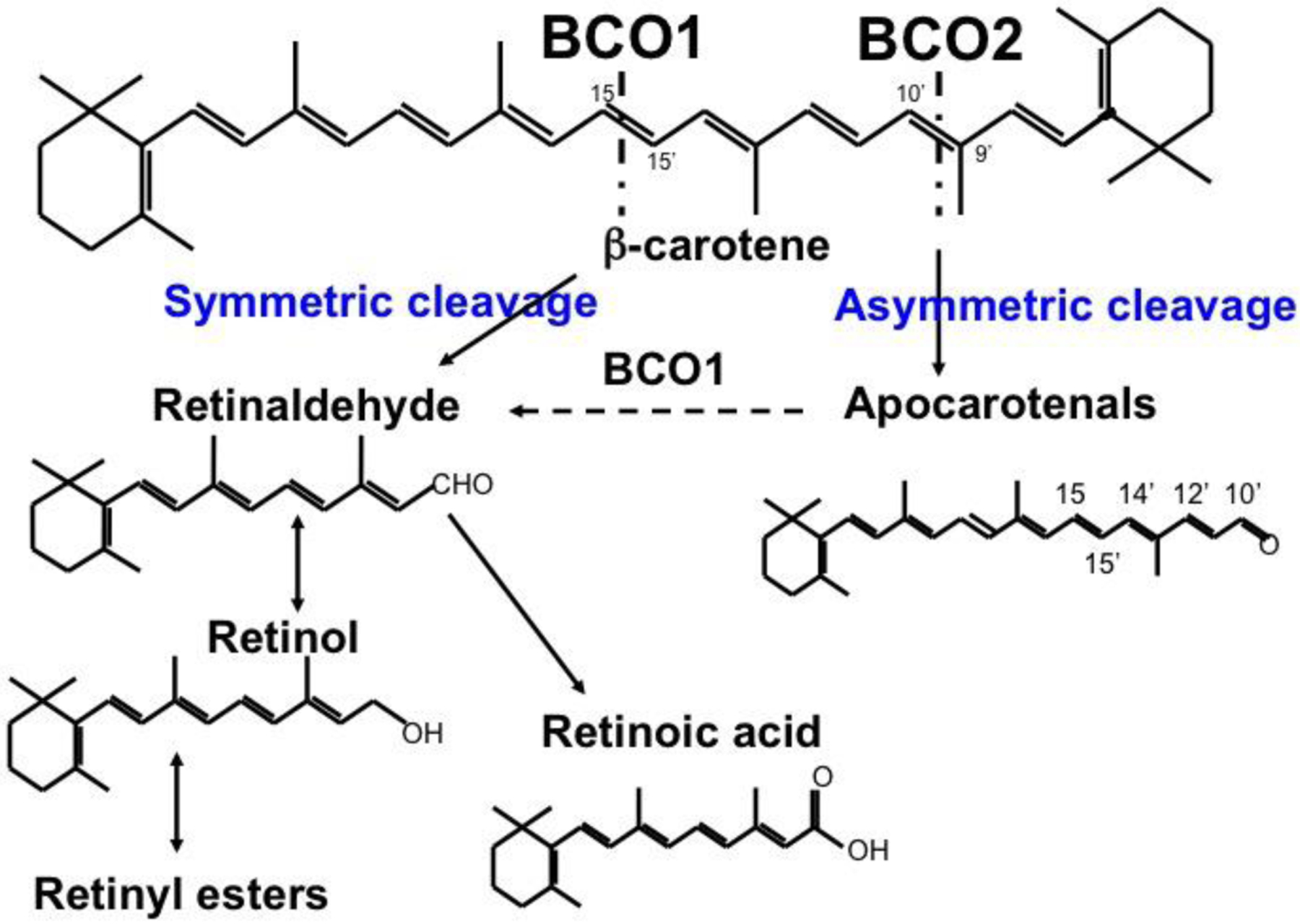
Central cleavage of β-carotene at the 15,15’ double bond is catalyzed by BCO1 to yield two molecules of retinal. Asymmetric cleavage at the 9’,10’ double bond is catalyzed by BCO2, and yields β-apo-10’-carotenal (depicted) and β-ionone (not shown). Retinaldehyde can be oxidized to retinoic acid or reduced to retinol, which can then be esterified to form retinyl esters.
