Figure 4.
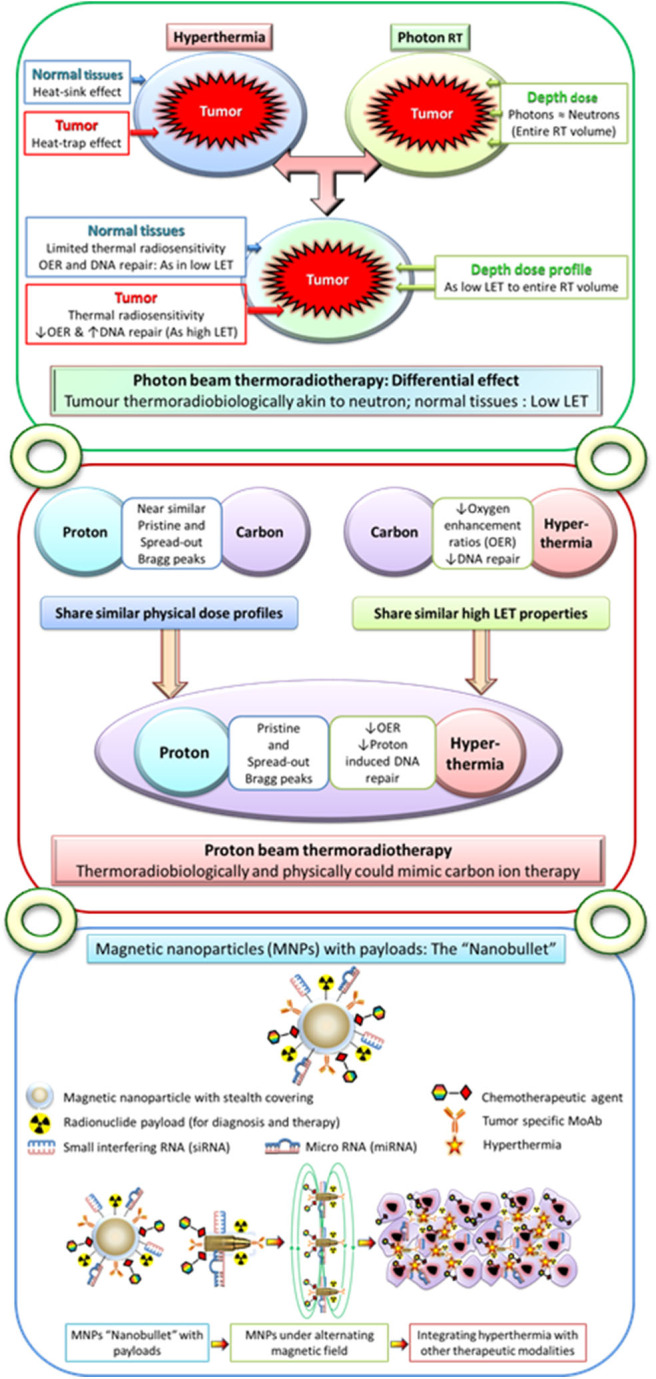
Upper panel: Moderate hyperthermia, with its ability to inhibit radiotherapy-induced DNA repair and its radiosensitizing effects on hypoxic tumor cells, has features akin to high linear energy transfer (LET) radiations. Following hyperthermia, the normal tissues exhibit a “heat-sink” effect resulting in the washing off the delivered heat due to heat-induced vasodilation and thus spared from thermal radiosensitization. On the other hand, tumors with its altered vasculature would fail to vasodilate and thus retain heat leading to a “heat-trap” effect. As the physical dose profiles of photons/X-rays are similar to those of neutrons in the irradiated volume, thermoradiobiologically photon beam thermoradiotherapy would have differential effects on tumors and normal tissues. This would be analogous to high LET neutrons for tumor owing to their selective thermal radiosensitization, whereas the normothermic normal tissues would be irradiated with low LET photons/X-rays. Middle panel: Protons radiobiologically have low LET radiation features but share similar physical dose profiles with those of high LET 12C ions. Thus, hyperthermia with its thermoradiological similarities to high LET radiations when delivered along with protons could mimic C12 ion therapy. Lower panel: Conceptual illustration of multifunctional magnetic nanoparticles with appropriate payloads of radioisotopes for radiotheranostics (both tumor imaging and radiotherapy) and chemotherapeutic and immunotherapeutic agents and siRNA and miRNA for therapeutic gene silencing. These magnetic nanoparticles in the presence of alternating magnetic field could deliver local hyperthermia and with additional payloads could act as “nanobullets.” The figure has been reproduced and modified with permission from Datta et al. (212, 213).
