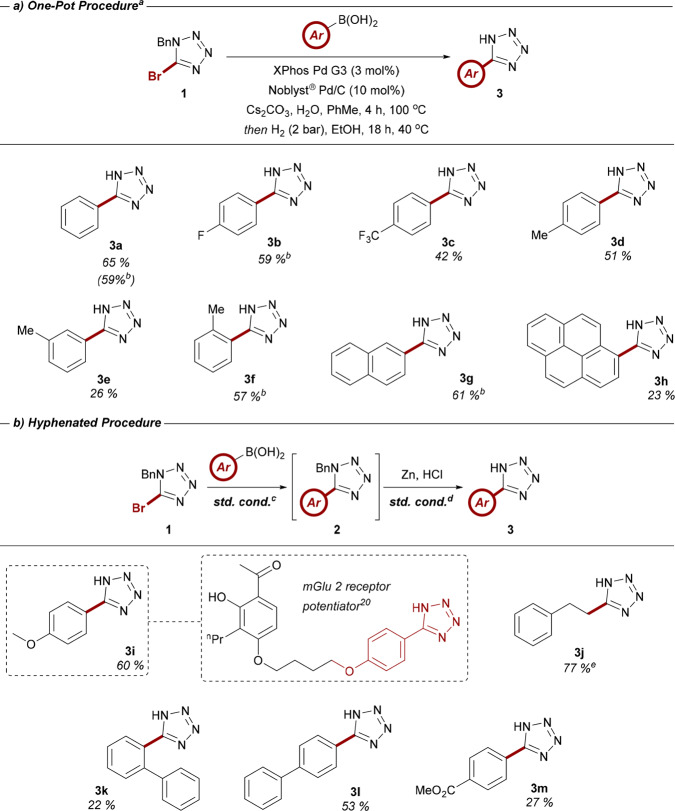
Table 5. (a) Exemplar Scope of Aryl Boronic Acids Compatible with the One-Pot Suzuki-Hydrogenolysis Coupling Protocol; (b) Library of Boronic Acids Employed as Part of the Hyphenated Two-Pot Synthesis of 5-Aryltetrazoles.
Reactions performed on a 0.5 mmol scale using 1.3 equiv of boronic acid, 1.5 equiv of Cs2CO3, and 100 equiv of H2O at a concentration of 0.1 M.
2.5 mmol scale.
1 equiv of 1, 1.5 equiv of boronic acid, 2 equiv of Cs2CO3, 3 mol % of XPhos Pd G3, and 10 equiv of H2O, heated at 100 °C in a solution of PhMe (0.2 M) for 2 h.
1 equiv of 3 and 10 mol % of Noblyst Pd/C, heated at 40 °C in a solution of EtOH (0.1M) for 18 h in the presence of 2 bar H2 generated from Zn and HCl.
From (E)-styrylboronic acid.