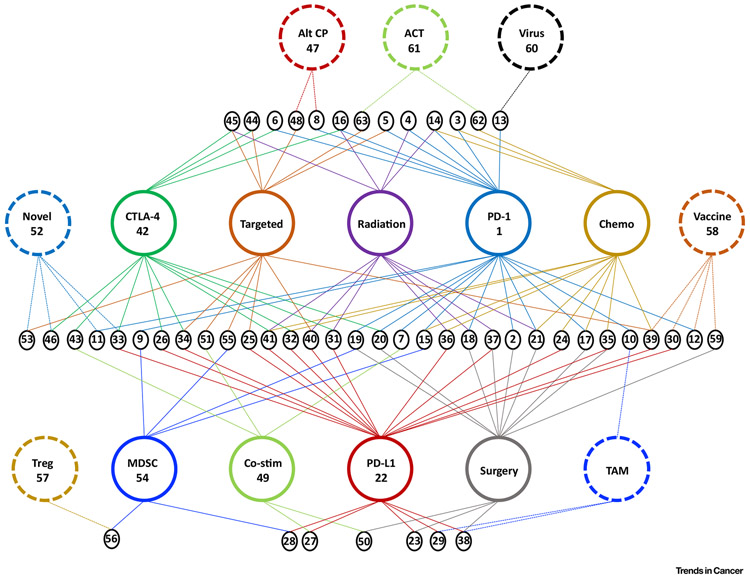
Figure 4. Combination Immunotherapy Clinical Trials in Head and Neck Cancer.
Of the nearly 200 clinical trials underway evaluating immunotherapy in head and neck cancer, the vast majority include combinations of therapy. Larger colored circles represent major treatment modalities in head and neck cancer. Smaller black circles at the nexus of multiple treatment modalities represent a unique combination treatment strategy under clinical trial evaluation. Numbers within the small black circles correspond to the numbers listed in the leftmost column of Table 1, where NCT numbers of active clinical trials (as of November 1, 2018) are listed. For example, number 45 in the upper left-most small black circle indicates the combination of CTLA-4 blockade with radiation and targeted chemotherapy and row 45 of Table 1 lists the corresponding clinical trials. ACT, Adoptive cell transfer; Alt CP, alternative checkpoint molecule; Chemo, chemotherapy; Co-stim, co-stimulatory pathway agonist; CTLA-4, cytotoxic T lymphocyte antigen 4; MDSC, myeloid-derived suppressor cell; Novel, novel immunologic treatment strategy; PD-1, programmed death 1; Targeted, target molecular therapy such as an epidermal growth factor receptor inhibitor; TAM, tumor-associated macrophage; Treg, regulatory T cell.