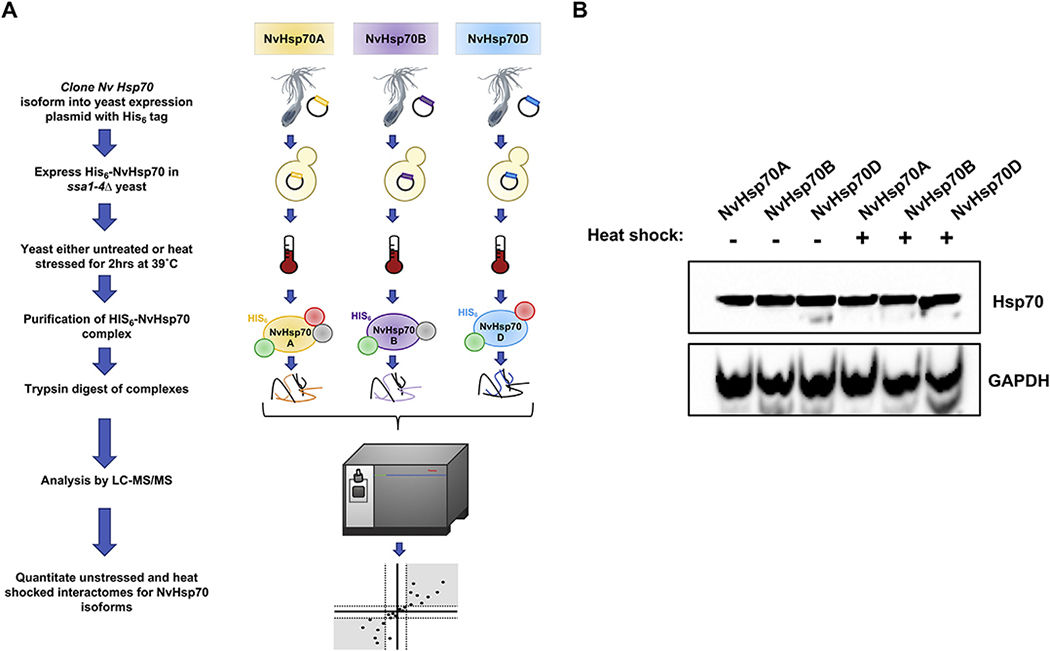
Fig. 1.
Quantitative affinity purification mass spectrometry for comparing NvHsp70 isoform interactions under heat stress. (A) NvHsp70 isoforms were expressed with an N-terminal 6xHIS tag in yeast lacking native Hsp70s. After exposing cells to heat stress (39 °C for 2 h), chaperone interactomes were isolated by nickel-NTA magnetic bead affinity purification. Following PAGE and in-gel trypsin digestion, peptides were analyzed by Orbitrap LC–MS/MS and MassQuant informatic analysis, allowing identification of interacting proteins and determination of their relative enrichment after heat stress. Each experiment was performed in biological triplicate. (B) Expression of NvHsp70 isoforms in yeast. Lysate of cells treated as in (A) were analyzed by SDS-PAGE/Western Blotting using pan-Hsp70 and GAPDH antibodies.